Chromatin, Epigenetics & Transcription
-

-
Trevor K. Archer, Ph.D.
Deputy Director, NIEHS;
NIH Distinguished Investigator -
Tel 984-287-4000
Fax 919-316-4566
[email protected] -
P.O. Box 12233Mail Drop B2-01Durham, NC 27709
Research Summary
Trevor K. Archer, Ph.D., is Deputy Director of NIEHS and head of the Chromatin and Gene Expression Group. The group studies chromatin remodeling complexes, epigenetics, and embryonic stem cell pluripotency.
The American Cancer Society predicts that there will be ~2 million new cancer cases and ~610,000 deaths in 2023. Thus, despite the recent declines in mortality (33%) and incidences from most cancers, cancer remains a potent and vital public health concern for the nation and central to the mission of the NIEHS (See the National Cancer Institute Cancer Trends Progress Report). Both the magnitude of the problem and the diversity of diseases that appear to comprise "cancer" have led to an intense increase in fundamental cancer research. While this research has developed along multiple avenues, efforts in the areas of chromatin & epigenetics, and stem cell biology have become particularly intense.
In human cells, DNA is organized with the aid of small basic proteins, histones, to form chromatin. The nucleosome, comprising 147 base pairs of DNA and 2 copies each of histones H2A, H2B, H3 and H4, is the fundamental organizing principle of chromatin. Nucleosome core particles are connected by a short stretch of linker DNA bound by histone H1, further stabilizing the nucleosome structure and higher-order chromatin architecture. It is this architecture that is the substrate for epigenetic regulation. Biological processes, including transcription must overcome this inherently repressive structure and they do so with the aid of two classes of enzymes. The first class disrupts chromatin structure using ATP-dependent chromatin remodeling, and the second class mediates the covalent modification of histone proteins. These enzymes and their activities often are altered in cancer cells, thus becoming important targets for novel therapeutics.
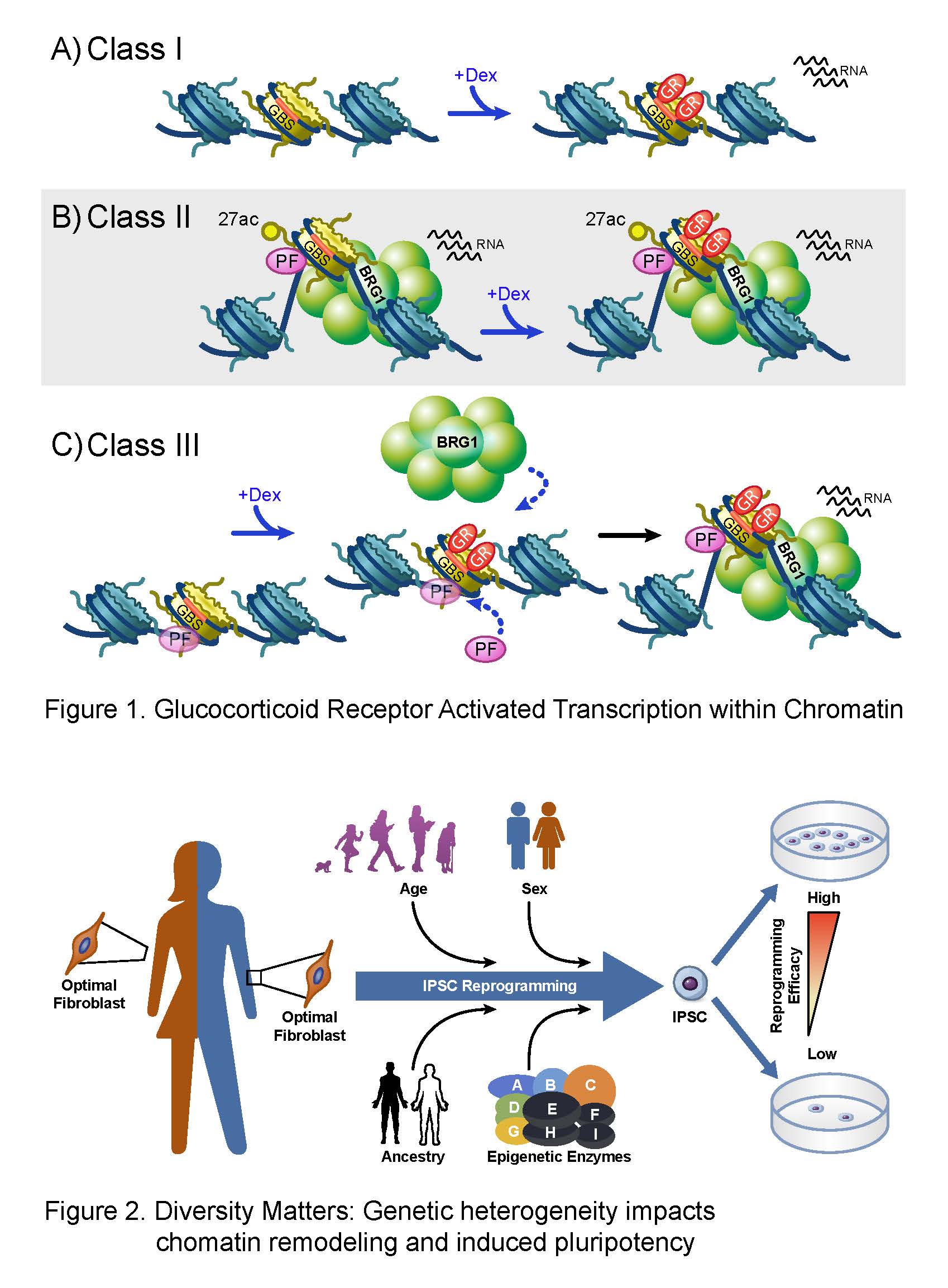
The development of human embryonic stem cells 25 years ago and the realization, that pluripotent stem cells could be derived from adult fibroblasts by introducing four transcription factors, ushered an exciting era in molecular medicine. The shared characteristic for continuous self-renewal and ability to differentiate into all cell types is thought to imbue these cells with great therapeutic potential. For this reason, the mechanisms that underpin reprogramming of the genetic repertoire of these cells represent attractive targets for chromatin and epigenetic studies.
Reprogramming of adult somatic cells to a pluripotent state has the potential to transform disease modeling and regenerative medicine. Specifically, these induced pluripotent stem cells, or iPSCs can self-renew and have the potential to differentiate into many different cell types, which makes them a promising source for developing unique and personalized disease and drug screening models. However, the process of generating iPSCs is highly inefficient, and the extent to which endogenous gene expression impacts reprogramming efficiency in individuals is largely unknown. What is known is that there is a clear requirement during this process for the silencing of lineage-specific genes and activation of pluripotency genes mediated by epigenetic mechanisms.
Archer earned his Ph.D. in 1987 at Queen’s University, Kingston Ontario Canada. He has published more than 60 peer-reviewed articles in leading biomedical journals as well as several book chapters. He served as a Tenured Associate Professor in the Departments of Biochemistry and Obstetrics and Gynecology at the University of Western Ontario in London Canada before joining NIEHS in 1999.
Major Areas of Research
Transcription, Chromatin and Epigenetics in Normal and Cancer Cells:
- To evaluate chromatin remodeling proteins and nuclear receptors in hormone dependent transcription and development.
Transcription, Chromatin and Epigenetics in Normal and Pluripotent Stem Cells:
- To explore how chromatin and epigenetics regulate human embryonic and induced pluripotency stem cell biological functions.
Current Projects
- What is the biological significance of the SWI/SNF (BRG1) chromatin remodeling complex in hormone dependent transcription and development?
- What is the function of histone H1 in hormone-dependent transcription?
- What epigenetic factors regulate pluripotency in human ES cells?
- What is the impact of age, sex, and ancestry on iPSC reprogramming efficiency?
- What epigenetic factors regulate iPSC reprogramming efficiency?
- How do environmental exposures alter hormone dependent transcription?
- Understanding BRG1’s role in murine early cardiac development and the adult heart
Experimental Model Systems
- Breast and other human cancer cell lines
- Human ES cells
- Diverse donor-derived healthy dermal fibroblasts and corresponding iPSCs
- Murine cancer cells
- Conditional knockout mouse models


