Repair & Stress Signaling

Research Summary
Michael A. Resnick, Ph.D., has been head of the Chromosome Stability Group for several decades, and holds a secondary appointment in the NIEHS Immunity, Inflammation and Disease Laboratory.
The Chromosomal Stability Group integrates mechanisms and genetic controls of genome stability with environmental factors and stress-signaling to better understand their complex contributions to human health. Using budding yeast and human cell models, the group has focused on genome maintenance and natural or environmental challenges to chromosome stability. Because of similarities in genome organization, enzymatic processes and genetic controls, findings with yeast are often applicable to human disease.
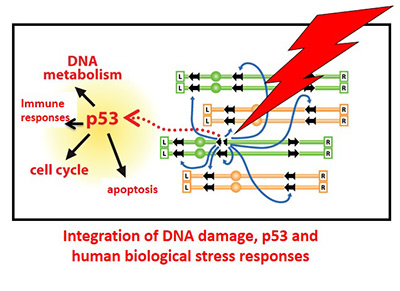
Repair, recombination, mutagenesis and checkpoint functions are investigated to understand sources of genome instability and mechanisms of coping with DNA damage, particularly double-strand breaks (DSBs). The functionality of human genes and networks are examined in these processes, with special emphasis on the prominent p53 tumor suppressor and stress responses. The Group has been deciphering the many roles that p53 plays in environmental stresses including modulation of immune responses and identifying agents that can influence components of the p53 network and are now extending their findings to RSV and HIV infection. The studies of DNA damage, repair and cell surveillance of its genome are directly relevant to human exposures, both endogenous and environmental, repair capabilities and therapies.
Major areas of research:
- Genetic and structure-function relationships in replication, repair and mutation avoidance
- Sources of double-strand breaks, genetic consequences and mechanisms of repair
- Organization and evolution of p53 tumor suppressor master regulatory network and consequences of mutations
- The role of the p53 network in inflammatory responses
- Environmental agents and conditions that impact genome stability
Current projects:
- Characterizing the expanding universe of p53 targets
- Determining p53 role in human immune/inflammatory systems, including the innate immune TLR (toll-like receptor) and Apobec3 family of genes and, within human primary and cancer cells.
- Assessing the role of p53 in HIV and RSV infection.
- Human variability in p53-associated stress responses
Overall, the CSG studies have resulted in novel mechanistic approaches to clinically relevant diseases that may have strong environmental components. These combined yeast and human cell approaches increase our understanding of environmental factors that put human genome stability and health at risk and provide opportunities for interventions.
Resnick received his Ph.D. from the University of California, Berkeley, and did postdoctoral work with the Medical Research Council in London and Oak Ridge National Laboratories. He held faculty positions at the U. Rochester, NY, the National Institute for Medical Research, London, and has been with NIEHS since 1979. He has authored approximately 200 peer-reviewed articles, 50 reviews and book chapters, and holds 7 patents. His group has received a “Best Paper of the Year” at NIEHS five times since its inception in 2003. He received the 2008 NIEHS Scientist of Year Award and was elected Fellow of the American Association for the Advancement of Science (AAAS) in 2012.
Relevance to NIEHS Mission
Many environmental factors put human health at risk through genomic destabilizing processes. The CSG is identifying relevant risks, mechanisms and biological consequences.


