Cellular Response to Genotoxic Stress

Paul W. Doetsch, Ph.D.
[email protected]
Research Summary
Paul W. Doetsch, Ph.D., is Deputy Scientific Director of NIEHS and head of the Mutagenesis and DNA Repair Regulation Group.
DNA damage from endogenous sources, such as reactive oxygen species produced by mitochondria during respiration, and environmental agents, including radiation and genotoxic chemicals is ubiquitous and, poses a constant threat to genomic integrity. How cells respond to genotoxic stress has a profound influence in determining the potential deleterious biological endpoints of DNA damage in humans, including cancer, neurodegenerative diseases and developmental disorders, as well as aging.
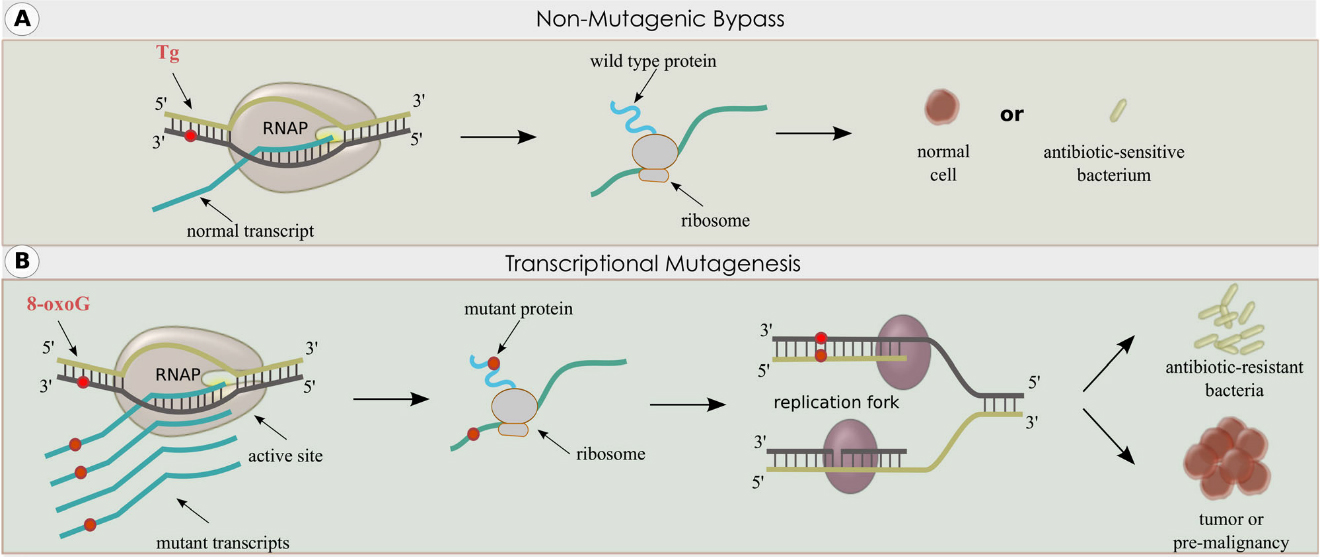
The Mutagenesis and DNA Repair Regulation Group’s major areas of research are the regulation of DNA repair and the interaction of the replication and transcription machinery with DNA damage. The group’s DNA repair studies are primarily focused on the base excision repair (BER) pathway and include the genetic and biological consequences, including tumorigenesis, dysregulation, as well as interactions of BER components with other repair systems. The group’s studies on the effects of various types of DNA damage on RNA polymerases led to the discovery of transcriptional mutagenesis (TM) several decades ago, establishing that TM occurs in bacterial and mammalian cells. If a phenotype caused by TM results in DNA replication or cell cycle entry, one of the resulting daughter cells may acquire a permanent DNA mutation, and thus permanent establishment of the phenotype. This mechanism has been termed retromutagenesis (RM). TM and RM may have a deleterious impact on human health by contributing to the etiology of diseases, such as cancer, as well as giving rise to antibiotic-resistant pathogenic bacteria. The Mutagenesis and DNA Repair Regulation Group is also conducting studies on the mutational signatures caused by redox stress in specialized DNA contexts and environments.
Doetsch received a B.S. in biochemistry from the University of Maryland, an M.S. in medicinal chemistry and pharmacognosy from Purdue University, and a Ph.D. in biochemistry from Temple University. Following postdoctoral training at the Dana Farber Cancer Institute and Harvard Medical School, he served on the faculty of Emory University School of Medicine for 32 years, led an active research group in the area of DNA repair and mutagenesis, and held various leadership positions including Associate Director for Basic Research (2005-2017) of the Winship Cancer Institute. He joined NIEHS January 2018.


