Laser Microdissection
Laser Microdissection provides the following information to NIEHS researchers interested in the process of microdissection.
Much of the work carried out by DTT is in support of the National Toxicology Program (NTP), an interagency partnership of the Food and Drug Administration, National Institute for Occupational Safety and Health, and NIEHS.
Discussion of Laser Microdissection Projects
Discussion of Laser Microdissection Projects
Since ideal tissue preparation parameters for mesenchymal tissues and epithelial tissues differ and because considerations of fixation protocols may vary depending upon the ultimate purpose of the microdissected samples, discussion of each project in advance is recommended.
Identifying Areas to Microdissect
Identifying Areas to Microdissect
Investigators should be aware that identification of structure in uncoverslipped slides is not as easy as on coverslipped preparations. Double-scoping potential samples on the morphology reference (Map) slide to identify areas for microdissection with a pathologist is recommended. Scheduled time with a pathologist may be arranged.
Optimal Tissue Collection for Laser Microdissection
Optimal Tissue Collection for Laser Microdissection
Frozen tissues are recommended for optimal recovery of DNA, RNA and protein.
- Harvest tissue samples within 5 minutes of animal death in a sterile environment (RNase free blades and instruments, wear gloves and clean work area).
- Place a metal disk or equivalent on dry ice to provide a flat work surface.
- Place appropriate size Tissue Tek® Cryomold® on top of the metal disk.
Squeeze a thin layer of OCT into the bottom of the mold and quickly orient the tissue (Figure 1). Label the Cryomold®.
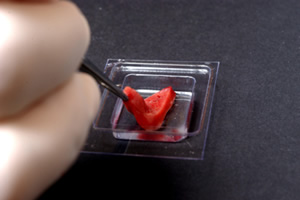 Figure 1: Orienting the tissue.
Figure 1: Orienting the tissue.
- Fill the Cryomold® to capacity and allow OCT to freeze completely (Figure 2). Label Cryomold® with a permanent marker.
 Figure 2: Fill and freeze mold.
Figure 2: Fill and freeze mold.
- Transfer samples to a labeled box or plastic bag and store at -80°C.
Snap freezing in isopentane can destroy tissue morphology and is not recommended for LM procedures
Laser Microdissection Cryostat Section Preparation
Laser Microdissection Cryostat Section Preparation
Section frozen tissue on a clean, Rnase-free cryostat (Figure 3). The study application determines how many slides to cut.
 Figure 3: Sectioning frozen tissue.
Figure 3: Sectioning frozen tissue.
Keep slides, with tissue sections, in a slide box on dry ice until all sectioning is completed (Figure 4).
 Figure 4: Slides on dry ice.
Figure 4: Slides on dry ice.
Frozen Tissue Storage
Frozen Tissue Storage
Store tissue and slides at -80°C. Frozen sections for RNA are stored at -80°C and should be used within one week of sectioning. It has been our experience that RNA degradation is significant in frozen tissue blocks removed from -80°C more than two times. For this reason, a frozen tissue block is not sectioned more than two separate times for a project.
Laser Microdissection Staining of Frozen Sections
Laser Microdissection Staining of Frozen Sections
Frozen sections are recommended for optimal recovery of DNA, RNA and protein. Information for reagents can be found on the Reagents & Supplies webpage.
- Remove slides from freezer and air dry for 30 seconds.
- 75% ethanol fix (1 minute)
- Nuclease-free water (15 seconds)
- 1% Cresyl Violet acetate ( 30 seconds) Filtered before every use!
- 75% ethanol (30 seconds)
- 95% ethanol (30 seconds)
- 100% ethanol (30 seconds)
- 100% ethanol (30 seconds)
- Air dry completely under hood (5 minutes)
Paraffin Block Preparation for Laser Microdissection
Paraffin Block Preparation for Laser Microdissection
Ten percent Neutral buffered formalin (18-24 hour) fixation of tissues can be used for isolation of DNA. Protocols may be optimized to isolate for RNA extraction. This is a future direction of the lab.
Laser Microdissection Paraffin Slide Preparation
Laser Microdissection Paraffin Slide Preparation
Slide type is dependent upon the laser platform used. The total number of sections cut depends on the study application. LM slides will be stained per protocol, and a total of seven slides may be prepared for each sample. The following steps are recommended procedures for DNA:
- Cut three slides for LM. Depending upon the size and number of structures present, all three slides may be needed for harvesting the sample. These slides are cut at five to eight microns.
- Slide four will be cut at five microns, placed on a charged slide, stained with routine H&E and coverslipped. This is the Map, morphology reference, slide.
- Slides five through seven will be cut at five to eight microns and left as uncleared paraffin sections. These are duplicate samples should there be insufficient material from the first three slides to allow for collection of serial samples.
If additional methods (IHC, ISH etc.) are desired on the LM sample, serial sections can be cut from the block at this time.
Laser Microdissection Staining of Paraffin Sections
Laser Microdissection Staining of Paraffin Sections
Paraffin embedded tissue should be cut per protocol. This procedure is optimal for DNA recovery, and reflects an NIH protocol that has been modified by NIEHS.
- Xylene (3 minutes)*
- Xylene (3 minutes)*
- 100% ethanol (30 seconds)
- 100% ethanol (30 seconds)
- 95% ethanol (30 seconds)
- Nuclease-free water (15 seconds)
- 1% Cresyl Violet acetate ( 30 seconds) Filtered before every use!
- 75% ethanol (30 seconds)
- 95% ethanol (30 seconds)
- 100% ethanol (30 seconds)
- 100% ethanol (30 seconds)
- Xylene (1 minute)
- Xylene (1 minute)
- Air dry completely (2-4 minutes)
*Excessive time in the xylene may cause the PEN or PET foils to separate from the slide frame.
Reagents & Supplies
Cresyl Violet acetate
- Sigma-Aldrich
800-325-3010
Catalog #C1791
Tissue Tek® Plastic Cryomold®
- Sakura Finetek
800-725-8723
Catalog #4557
Tissue Tek® OCT Compound
- Sakura Finetek
800-725-8723
Catalog #4583
This content is available to use on your website.
Please visit NIEHS Syndication to get started.


