The Human Research Protection Program (HRPP) provides essential services for NIEHS investigators to support compliance with laws, regulations, policy, and guidance related to human subjects research. HRPP is comprised of credentialed regulatory experts who help to ensure sound research design and scientific integrity, and they foster a research environment that contributes to generalizable scientific and clinical knowledge.
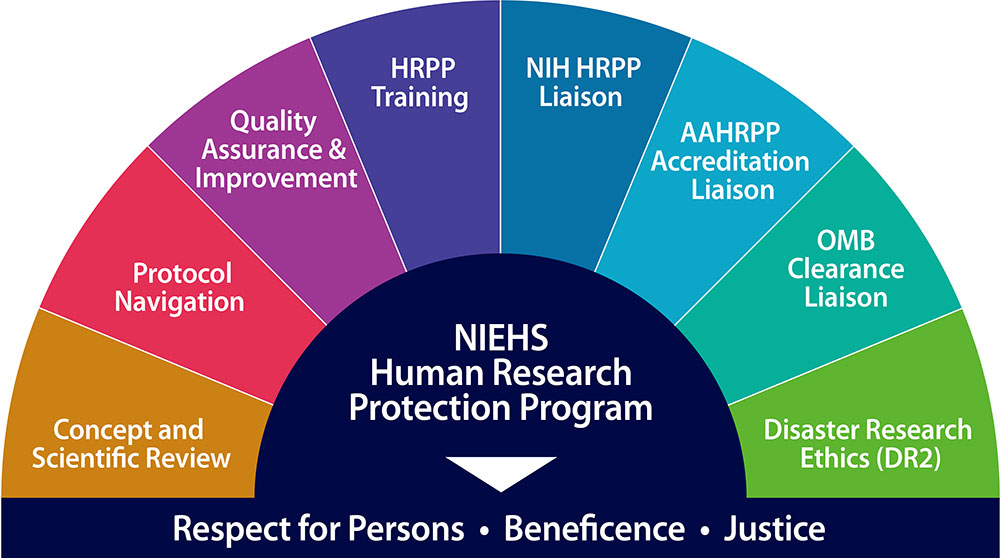
HRPP, which is part of the NIEHS Office of Human Research and Community Engagement, ensures compliance with all applicable regulations and policies governing human subjects research and serves eight essential functions for the institute.
- NIEHS Clinical Advisory Committee (CAC) and Scientific Review Committee (SRC) Administration: Manages and administers these processes for NIEHS. The CAC provides feedback to investigators in creation of their protocols, and the SRC includes the initial, annual, and quadrennial review of NIEHS protocols.
- Protocol Navigation: Supports leadership, clinical investigators, and study staff by providing management, regulatory guidance, technical writing, training, and review for the preparation and submission of clinical research protocols to the CAC, SRC, and NIH Institutional Review Board (IRB).
- Quality Assurance and Quality Improvement: Conducts internal and external audits of NIEHS clinical studies and initiates corrective and preventative action (CAPA) reports, in addition to reviewing and summarizing monitoring reports for NIH.
- HRPP Training: Conducts weekly meetings and individualized training sessions to ensure investigators and study staff receive updates and are trained on human protections policies, procedures, best practices, and standards in the conduct of human subjects research.
- NIH HRPP Liaison: Serves as the NIEHS regulatory expert for the NIH Office of Human Subjects Research Protections (OHSRP), NIH Office of Research Support and Compliance (ORSC), and NIH Office of Protocol Services (OPS), representing NIEHS interests within the NIH HRPP.
- AAHRPP Accreditation Liaison: Provides data for annual reporting requirements and facilitates the NIH Association for the Accreditation of Human Research Protection Programs, Inc., (AAHRPP) reaccreditation processes, including site visit preparation and community engagement initiatives.
- NIEHS Paperwork Reduction Act (PRA) Liaison for Office of Management and Budget (OMB) Clearance: Facilitates clearances for NIEHS surveys and information collection activities.
- NIH/NIEHS Disaster Research Response (DR2) Regulatory and Community Outreach Program: Within the DR2 program, OHRCE has led the national effort to improve IRB preparedness for reviewing disaster-related research. OHRCE HRPP experts have helped to create guidance and tools for best practices and special considerations for IRB review. They have also trained IRBs and investigators in the use of these tools as well as in effective community engagement with vulnerable populations, pre- and post-disaster.