Superfund Research Program
With support from the NIEHS Superfund Research Program (SRP), researchers at the University of Arizona and the small business GlycoSurf are pioneering strategies to balance the mining of important resources with sustainable and health-protective approaches. Their biosurfactant technologies — based on compounds produced by microorganisms — offer a safe and cost-effective solution to simultaneously clean up mining waste and recover valuable rare earth elements.
The Problem
Uranium mining in the U.S. Southwest has left a legacy of uranium-contaminated soils that pollute nearby water and land, posing significant health and environmental risks. Additionally, toxic metals like lead, cadmium, and arsenic, along with rare earth elements, often contaminate mining waste, complicating remediation.
Traditional methods for treating these contaminants have limitations. For example, chemical precipitation is inefficient, especially for low metal concentrations, and generates large amounts of waste sludge. Membrane filtration, though effective, is expensive and prone to clogging, making it impractical for widespread use. As a result, mining wastewater is often stored or discharged into surface waters, leading to ongoing environmental harm.
SRP Solutions

Maier was the director of the University of Arizona SRP Center from 2012 to 2025. She now serves as the center’s associate director and leads a research project. (Photo courtesy of the University of Arizona)
For nearly three decades, researchers at the University of Arizona SRP Center have explored the use of biosurfactants to tackle contamination in soil and water.
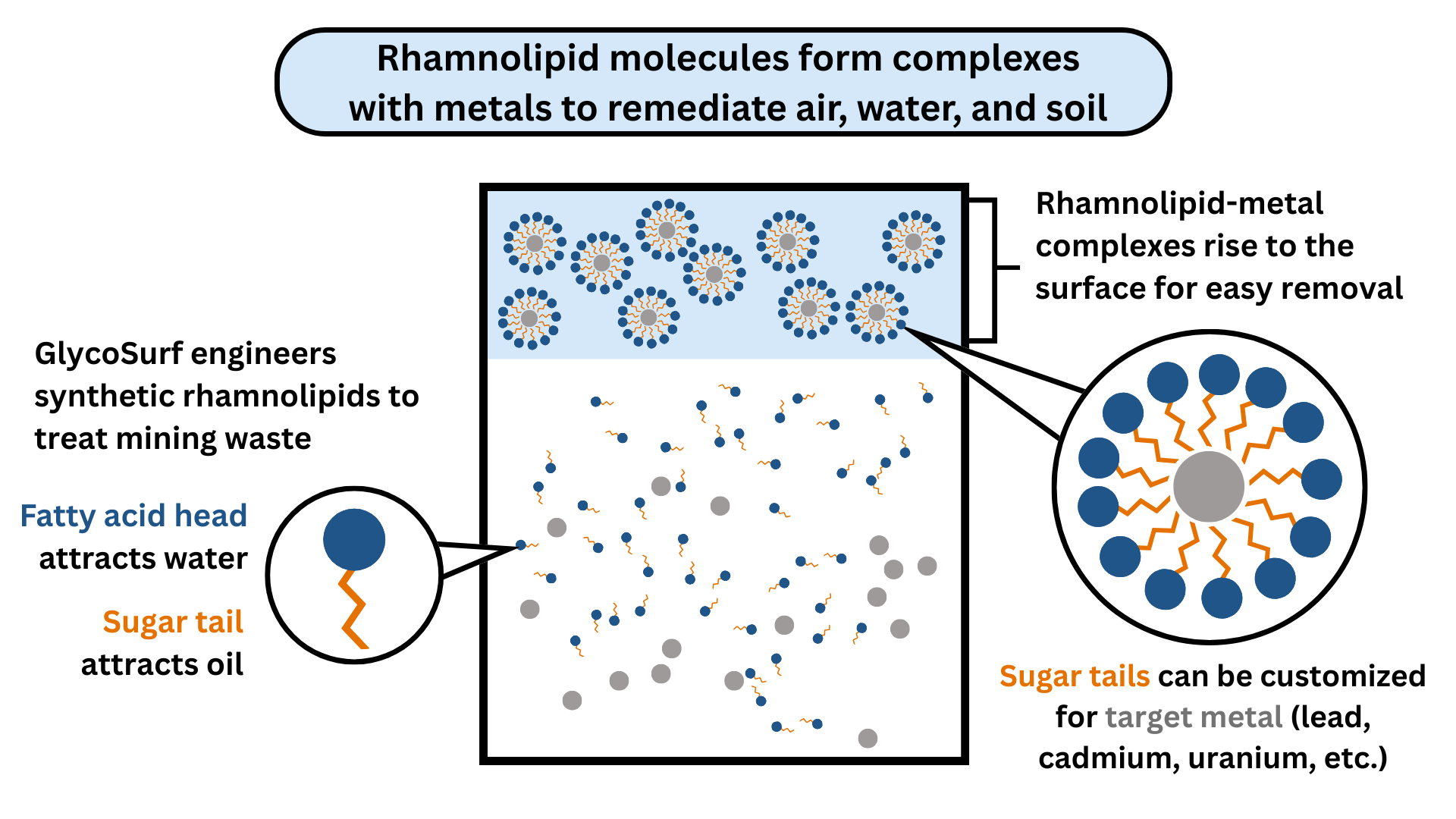
In 1995, Raina Maier, Ph.D., and her team found that a biosurfactant produced by the bacteria Pseudomonas aeruginosa could effectively bind cadmium, lead, and zinc and remove them from contaminated soil samples. Known as rhamnolipids, these molecules are made of a sugar and a fatty acid, giving them a distinctive structure with a water-attracting head and an oil-attracting tail. This dual nature enables them to interact with a wide range of pollutants, either lifting them from soil or binding them in water for easy removal. Because of their cleaning action, surfactants like rhamnolipids are often described as “soap-like molecules,” capable of removing contaminants much like soap washes away grease.
Maier and collaborators, including then-graduate student Francisco Ochoa Loza, Ph.D., later discovered that the effectiveness of rhamnolipids hinged on the ionic strength of the solution — a measure of how many charged particles, or ions, are present. By adding salts to adjust the ionic strength, they significantly boosted the rhamnolipids’ performance, allowing the team to scale up from simple batch experiments to more realistic simulated field conditions. Scaled-up experiments demonstrated that rhamnolipids could remove up to 80% of cadmium from soil, even in the presence of elements that typically hinder remediation efforts, like calcium and magnesium.
“We were surprised at just how strongly these materials could bind metals. That initial finding lit a fire that never went out,” Maier said.
Soon after, the team began exploring broader applications for rhamnolipids. In 1997, Maier and team discovered that rhamnolipids can be used to rapidly kill fungus-like organisms that damage crops. The researchers patented the rhamnolipid fungicide and licensed the patent to a biosurfactant company in September 2004. The rhamnolipid fungicide is registered for use with the U.S. Environmental Protection Agency (EPA).
Over the next decade, the University of Arizona team continued to study surfactants, deepening their understanding of how they are formed, establishing their rhamnolipids’ effectiveness for remediating lead-contaminated soils, and even finding a new class of surfactants, called flavolipids, that can degrade organic contaminants.
Pathway to Innovation
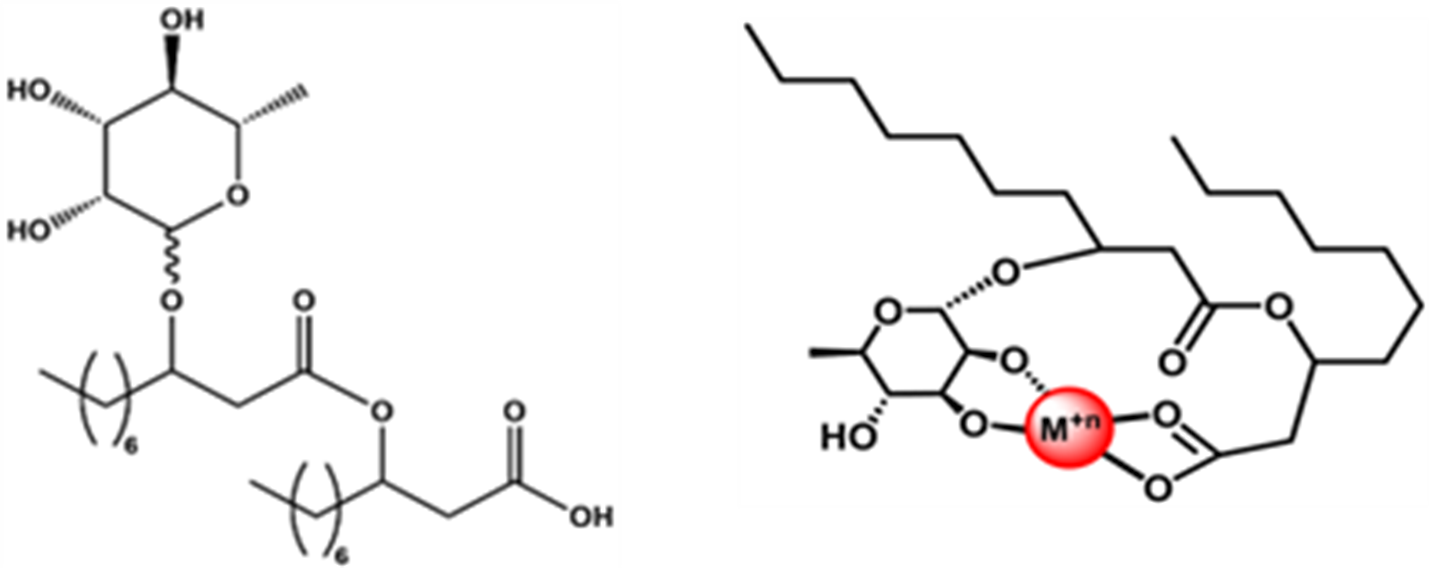
(Left) Monorhamnolipid C10-C10. (Right) Monorhamnolipid C10-C10 shown with a metal (in red) in the metal-binding pocket. (Image courtesy of GlycoSurf)
Building on the success of this foundational research, Maier and collaborator Jeanne E. Pemberton, Ph.D., co-founded GlycoSurf in 2013. They recruited University of Arizona alumnus Chett Boxley, Ph.D., as CEO a few months later. Their goal was to commercialize sugar-based surfactants, including rhamnolipids, by developing a proprietary synthetic process that offered control, consistency, and scalability.
“In nature, rhamnolipids are produced via microbial fermentation, but the product is typically a complex mixture of molecules with different chain lengths and sugar units,” Boxley explained. “That variability limits industrial use. Our aim was to build a synthetic platform that retains rhamnolipids’ natural power while giving us the control needed for specific applications.”

Bobby Bruggeman, senior chemist at GlycoSurf, in the laboratory synthesizing glycolipids. (Photo courtesy of GlycoSurf)
Instead of fermentation, GlycoSurf uses chemical synthesis to custom-design rhamnolipids with precisely controlled sugar types, fatty acid chains, and molecular structure. This approach allows them to tailor surfactant properties for targeted uses, such as metal removal in wastewater or remediation of contaminated soils.
The company creates the molecules and works with Maier and research assistant professor David Hogan, Ph.D., a former Maier student who joined the project in 2011, to test them at the University of Arizona.
“As a start-up, the company was a true leap of faith,” Boxley said. “We had the science, but nothing else — no funding, no recognition — just the idea that this could work.”
Recovering Metals from Wastewater
In 2018, GlycoSurf received its first Small Business Innovation Research (SBIR) Grant, funded by SRP, to explore the use of rhamnolipids and ion flotation to recover rare earth elements from mining wastewater. Ion flotation uses air bubbles to carry metal-surfactant complexes to the surface of a solution, where they can be skimmed off.
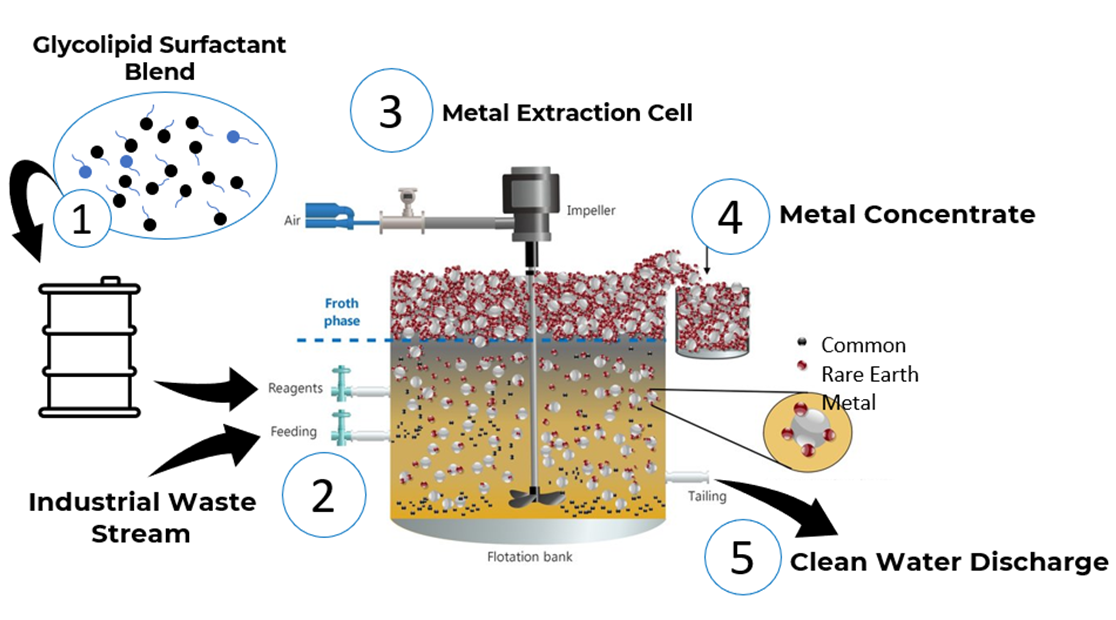
This graphic depicts the process of extracting metals from water via ion flotation. (Image courtesy of GlycoSurf)
"Rare earth elements are used in consumer electronics, renewable energy technologies, and national defense, but traditional mining is resource-intensive and environmentally damaging,” said Hogan. “Recovering these metals from waste streams like brines or mine drainage could be a cleaner alternative, but we need selective, cost-effective extraction methods to make it viable.”
In 2023, the team tested seven different synthetic rhamnolipids and found they could extract over 97% of lead and lanthanum from contaminated samples. Lanthanum is a rare earth metal used in batteries for hybrid cars, fluorescent lighting, and x-ray detectors.
“Our technology not only cleans the water, but turns waste into a resource,” Boxley said.
A separate SBIR grant in 2020 expanded their efforts to include uranium-contaminated water. Using samples from the Monument Valley site, a former uranium mill on Navajo Nation land, the team achieved 92.6% uranium removal — lowering contamination to below EPA health-protective limits.
Long-Standing Partnerships with Mining Companies

“We hope to accelerate the shift toward cleaner, more sustainable resource extraction,” Hogan said. (Photo courtesy of the University of Arizona)
A cornerstone of success for GlycoSurf and the University of Arizona SRP Center is their deep, long-term collaboration with the mining industry. These relationships have allowed researchers to better understand the challenges faced in real-world operations and to design biosurfactant technologies that are both practical and scalable. Over the years, the team has worked closely with mining companies across the Southwest to test their materials under site-specific conditions.
“These relationships are crucial,” said Boxley. “They help us understand the day-to-day challenges mining companies face and allow us to design biosurfactants that are effective, durable, and compatible with existing operations.”
Much of this collaboration has been facilitated by the Center for Environmentally Sustainable Mining, co-founded by Maier in 2011. The center’s advisory board includes mining companies throughout the Southwest U.S., who share their ideas, needs, lessons learned, and best practices.
Cleaning Up Air

Laboratory wind tunnel testing apparatus. A plate containing mine tailings either moistened with water or with biosurfactant solution is placed inside the tunnel. Air is blown over the surface and the sensor in the lid of the container quantifies small particulate matter. (Photo courtesy Damask Grinnell)
In 2022, GlycoSurf received an SRP SBIR grant to expand the use of their biosurfactants to address airborne pollution from mining, particularly dust generated during excavation, transport, and processing of metals. Mining-related dust accounts for around 12% of global particulate matter health impacts, contributing to respiratory illnesses and other serious conditions.
To address this, GlycoSurf developed a sustainable dust suppressant by combining their rhamnolipids with plant-based polymers. The result is a durable, non-toxic formulation that performs as well as conventional dust control agents, without harming the environment or damaging mining equipment. Once applied, the product remains effective through weather exposure and gradually biodegrades with rain over time, reducing long-term environmental impact.
In the second phase of this SBIR grant, funded in 2025, the team will start testing their technology on an active mining site.
“We’re not just trying to suppress dust,” Maier explained. “We’re trying to do it without polluting the water or damaging the soil.”
Crossing the Valley of Death: From Lab to Market
Bringing scientific innovation to market often means crossing the “valley of death”— the gap between academic discovery and commercial viability. GlycoSurf’s journey across that gap was made possible by technical innovation, persistent research, and the sustained backing of the SRP and other funding from the National Science Foundation and the Department of Energy.
Over the past three years, the company has dramatically scaled up.

“The SBIR program has been essential,” said Boxley. “It’s the reason we’re here. It’s how a molecule in a flask became a company with real-world impact.” (Photo courtesy of GlycoSurf)
"We’ve gone from making 10 grams in the lab to batches approaching 10 kilograms,” said Boxley. “Right now, we’re producing hundreds of grams each week and expect to hit kilogram-scale weekly production soon.”
This production growth supports the company’s goal of supplying biosurfactants for a variety of water treatment applications and ultimately making them widely available.
“By bridging the gap between academic research and real-world deployment, we hope to bring sustainable, science-backed solutions from the lab bench to communities across the world,” Maier said.


