The OTT has negotiated and executed many agreements that lead to inventions that are patent pending or allowed collaborative research on topics relevant to national and global public health. Below we highlight some of the recent technology transfer successes facilitated by the OTT.
NIEHS Scientists Innovate to Develop Improved COVID-19 Diagnostics
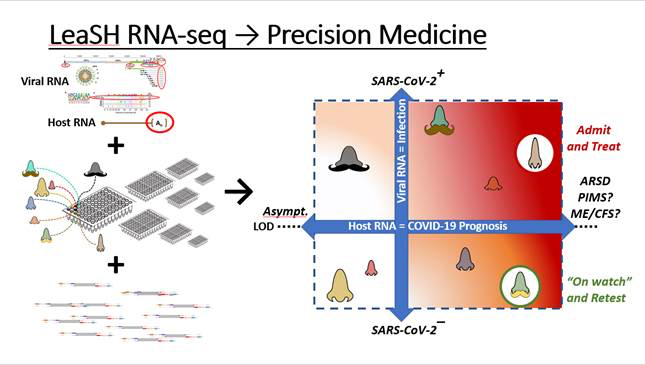
NIEHS scientists received $1 million from the Rapid Acceleration of Diagnostics (RADx) program to develop a method to scale up COVID-19 diagnostic testing.
The LeaSH-RNA-seq method uses sample-specific barcoded indexes that detect both SARS-COV-2 virus and the host’s transcriptional response to infection. This precision medicine focused method provides the capability for testing tens of thousands of patient samples in a large bolus, allowing accurate and fast-turnaround SARS-CoV-2 testing capacity at population scale while permitting massive scale monitoring of at-risk individuals with minimal processing delays. The OTT filed a provisional patent application on the LeaSH-RNA-seq diagnostic method to spur commercialization efforts and negotiated several CDAs, and eventually RCAs, to support clinical development of this technology.
CRADA Tests the Use of Hyaluronan to Prevent and Treat COVID-19
COVID-19 causes severe lung inflammation with long term ramifications in a subset of patients. Therefore, there is an urgent need for therapeutics that can mitigate severe COVID-19 symptoms. In May 2020, researchers at NIEHS identified several hyaluronan binding domains in the spike protein of SARS-CoV-2, the virus that causes COVID-19. Hyaluronan is an abundant extracellular matrix component of the lung known to have anti-inflammatory properties. This prompted the investigators to ask whether hyaluronan could be used as a COVID-19 therapeutic. In July 2020, the OTT negotiated and executed a Cooperative Research and Development Agreement (CRADA) with IBSA Institut Biochimique SA to test whether sodium hyaluronate can mitigate the pathophysiological effects of the novel coronavirus SARS-CoV-2.
NIEHS Joins the COPE Consortium
The rapid pace of the COVID-19 pandemic presents challenges to the real-time collection of population-scale data to inform urgent and evolving public health needs. The Coronavirus Pandemic Epidemiology (COPE) Consortium was formed to leverage the power of epidemiological cohort study data with new COVID-19 data provided by cohort study participants. The COPE Consortium developed a COVID Symptom Study mobile application as a common data collection tool for epidemiologic cohort studies with active study participants. The OTT swiftly negotiated a consortium agreement to ensure NIEHS had access to COVID-19 data obtained early in the pandemic. By linking NIEHS cohort data to data obtained from the COVID Symptom Study mobile application, NIEHS plans to identify health and environmental factors that affect the likelihood of developing COVID-19 and learn if the virus has any long-term health impacts.



