Summary Report on Request for Information (RFI) Responses
Use of In Vitro Lung Models in Inhalation Toxicology Research with Potential Application to Regulatory-Decision Making [NOT-ES-24-007 (FR)]
Authors
William Gwinn, Ph.D. and Kristen Ryan, Ph.D.
Occupational and Inhalation Exposures Program
Division of Translational Toxicology (DTT), NIEHS, NIH
Background
The Occupational and Inhalation Exposures (OIE) Program is one of the Exposure-Based Research Programs within the DTT. The problem statement for the OIE Program is: “Hazard characterization of inhalation exposures is critical to creating a safe living/working environment and reducing disease burden”. The OIE Program has three objectives to:
- Assess health hazards of airborne or occupational substances
- Expand capabilities for predicting adverse health effects
- Enhance the translational relevance of experimental models
The primary goal for objective 2 is two-fold:
- To predict adverse human health effects to the airways/lungs
- To generate data to support health hazard assessments or risk assessments through the evaluation and use of human-relevant in vitro approaches, including air-liquid interface (ALI) tissue models and other microphysiological systems (MPS) like lung-on-a-chip
The OIE Program released the Request for Information (RFI) entitled Use of In Vitro Lung Models in Inhalation Toxicology Research with Potential Application to Regulatory-Decision Making. The objectives of the RFI were to query key stakeholders and learn what are some of the key scientific knowledge gaps as well as challenges and limitations currently associated with the use of these in vitro lung models. The OIE Program envisioned that input from this RFI would help to inform the future directions for these models in research and potentially guide new projects, depending on what gaps and challenges/limitations were identified.
Methods: The RFI was publicly available on April 24, 2024, via both the NIEHS webpage (for the OIE Program) and the Federal Register [NOT-ES-24-007 (FR)]. The RFI asked for responses to these three questions:
Questions:
- Have you used or are you currently using in vitro lung models (preferably together with physiologically relevant in vitro inhalation exposure systems/technologies) for applications to [select all that apply]:
- Inhalation/Respiratory toxicity screening?
- Chemical-specific mechanistic studies?
- In vitro to in vivo extrapolation (IVIVE)?
- Development/validation for regulatory decision-making or risk assessment?
- What do you perceive are the most important scientific knowledge gaps underlying the assessment of the effects of inhaled substances (particles, vapors, etc.) on human health when utilizing in vitro lung models? [free text response]
- What do you perceive are the most important current technical challenges and limitations related to using in vitro lung models in research? [free text response]
RFI responses (without personally identifiable information) were collected for 6 weeks (with an end date of June 7, 2024). Additional response time was granted upon request. In addition to responses to the questions, some responders shared related materials such as publications or reports. Responses were received from both U.S. and international entities and from multiple sectors including academia, government, industry, and non-profit organizations.
DTT staff reviewed the responses. For Questions 2 and 3, the responses were evaluated and categorized/binned by similarity into major themes or groupings.
Results: DTT received RFI responses from 44 unique responders. Some of the responders were from the same organization. Not all responders answered every question. The number of responders for Questions 1 – 3 are shown in the graphs (Figures 1 – 3) below with descriptions in the figure legends.
Conclusions: This RFI provided the OIE Research Program valuable information regarding the current usage by stakeholders of in vitro lung models and helped to identify perceived critical knowledge gaps, challenges, and limitations to their use. Collectively, the information from this RFI will help guide areas for future research. The information will also assist the OIE Research Program in understanding better some of the challenges stakeholders face with these models and facilitate future discussions and the planning of potential workshops on this topic. Most responders currently use in vitro lung models for respiratory/inhalation toxicity screening, although many also use them for mechanistic studies, IVIVE modeling, and/or have a goal to develop/validate the models for regulatory decision-making purposes (Question 1). Responses overlapped regarding what are considered the most important scientific knowledge gaps (Question 2) and technical challenges/limitations (Question 3) associated with the current use of in vitro lung models for human-relevant inhalation toxicology research. This overlap included concerns over dosimetry, physiological/mechanistic relevance (model complexity, or lack thereof), validation of the models, and translational relevance/prediction (correlating in vitro model findings to data in vivo and in exposed humans). For Question 3, most responders identified reproducibility (including data variability and method standardization) as the top challenge/limitation in the use of these models.
Question 1.
Have you used or are you currently using in vitro lung models (preferably together with physiologically relevant in vitro inhalation exposure systems/technologies) for applications to [select all that apply]:
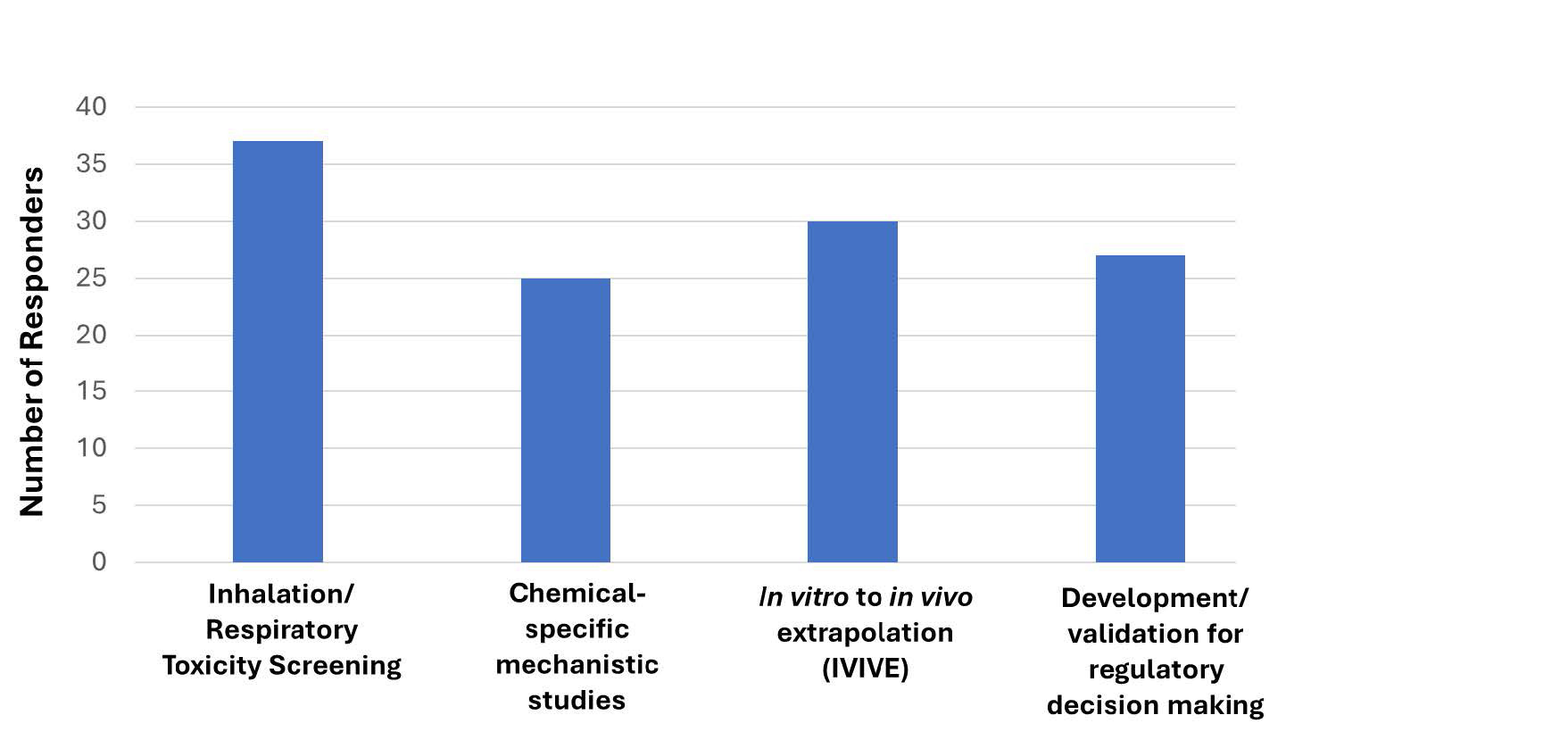
Figure 1. 44 responders answered Question 1. With the optionality to select 1 or all options, most responders (37 of 44) indicated that they are currently using in vitro lung models for inhalation/respiratory toxicity screening. 25, 30, and 27 responders are utilizing in vitro lung models for mechanistic studies, IVIVE, and/or to develop/validate (these models) for regulatory decision-making purposes, respectively.
Question 2.
What do you perceive are the most important scientific knowledge gaps underlying the assessment of the effects of inhaled substances (particles, vapors, etc.) on human health when utilizing in vitro lung models?
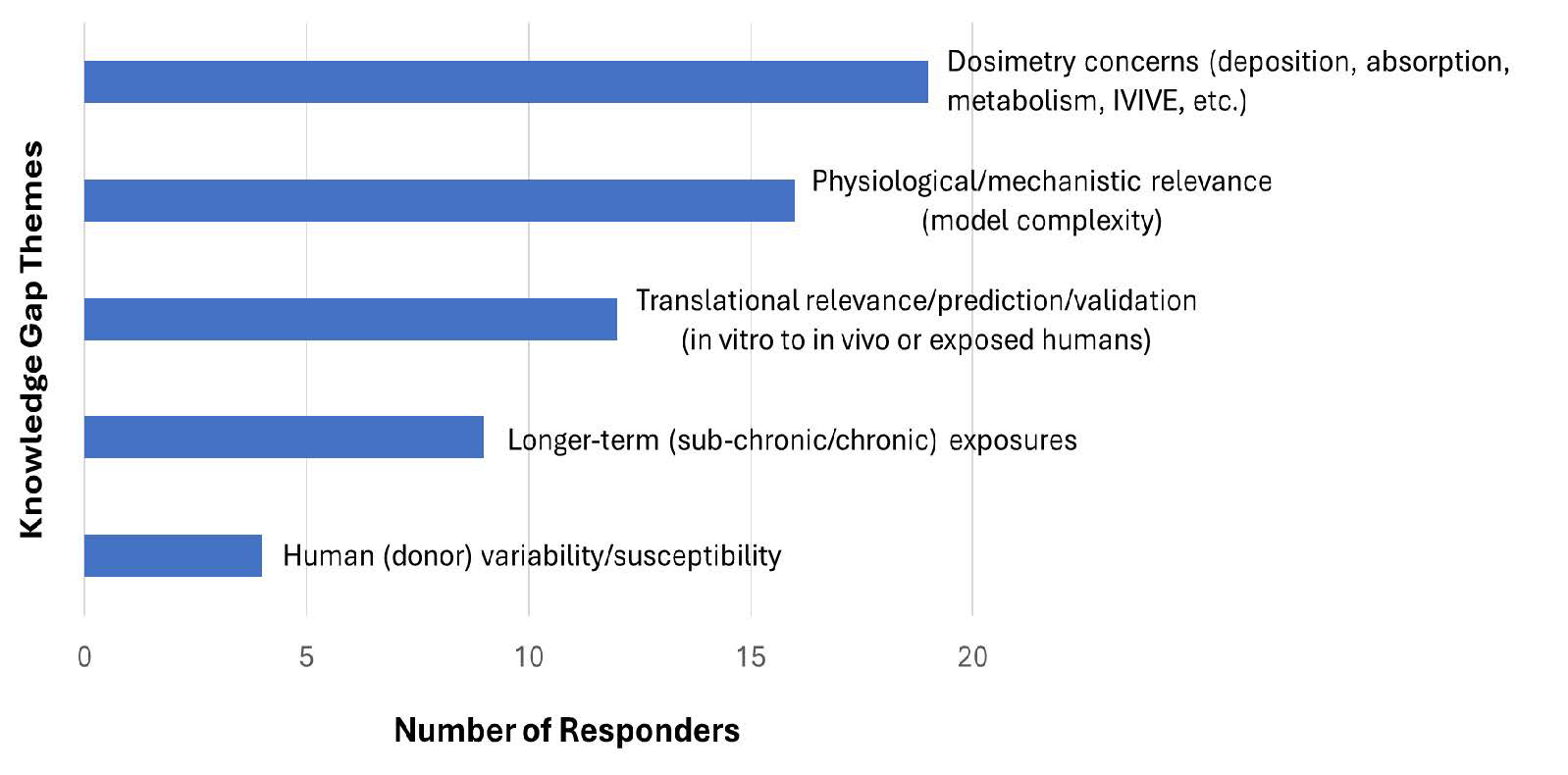
Figure 2. 27 responders answered Question 2, which had the option of responders providing multiple responses regarding the most important knowledge gaps. The responses were evaluated and categorized/binned by similarity into major themes or groupings.
- 19 responders identified the assessment of dosimetry as a key knowledge gap including deposition, absorption, metabolism, and IVIVE.
- 16 responders identified physiological/mechanistic relevance (model complexity, or lack thereof) which includes the absence of immune cells, tissue fibrosis (and remodeling), and systemic toxicity.
- 12 responders identified translational relevance/prediction and validation including the ability to correlate in vitro model findings to data in vivo and in exposed humans.
- 9 responders identified the capability to conduct longer-term (sub-chronic or chronic) exposure studies.
- 4 responders identified the consideration and incorporation of human (inter-donor) variability and susceptibility in these models.
Question 3.
What do you perceive are the most important current technical challenges and limitations related to using in vitro lung models in research?
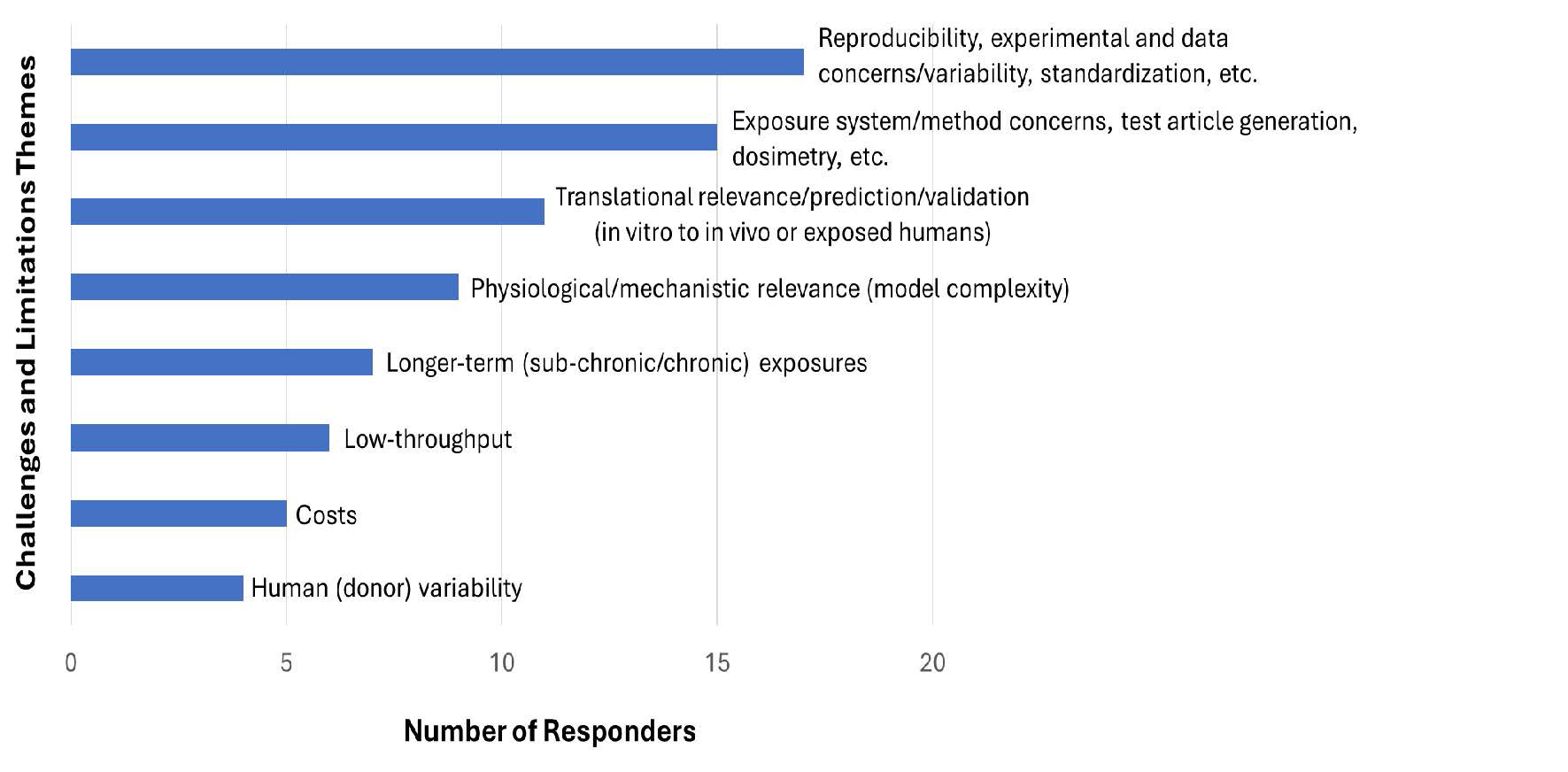
Figure 3. 25 responders answered Question 3 which had the option of responders providing multiple responses regarding the most important current technical challenges and limitations. The responses were evaluated and categorized/binned by similarity into major themes or groupings.
- 17 responders identified the concern over reproducibility (including data variability and method standardization) as a major challenge/limitation.
- 15 responders identified concerns over issues such as the exposure system/method, test article generation, and dosimetry.
- 11 responders identified translational relevance/prediction and validation including the ability to correlate in vitro model findings to data in vivo and in exposed humans.
- 9 responders identified physiological/mechanistic relevance (model complexity, or lack thereof) which includes the absence of immune cells, tissue fibrosis (and remodeling), and systemic toxicity.
- 7 responders identified the difficulty in conducting longer-term (sub-chronic or chronic) exposure studies.
- 6 responders identified the low throughput of these in vitro models.
- 5 responders identified the excessive costs associated with using these models.
- 4 responders identified the consideration and incorporation of human (inter-donor) variability in these models.


