Mechanisms of Placental Development and Function

Research Summary
Carlos M. Guardia, Ph.D. is head of the Placental Cell Biology Group and holds a secondary appointment in the NIEHS Molecular and Cellular Biology Laboratory and Neurobiology Laboratory. The group explores basic science mechanisms to understand the effect of stressors present in the environment on human pregnancy, with a particular focus on placenta development, structure-and-function studies of placental specialized processes, and in vitro placenta modeling.
Despite its essential role in fetal growth, the human placenta remains one of the least understood organs. Placentas that develop and function under stress are prone to cause serious pregnancy complications. Thus, autophagy mechanisms are in place to protect the placenta and promote its normal development and function. Failure or dysregulation of these pathways could explain the origin of several placental disorders, but also provide a window for new therapies.
Inactivation of autophagy genes in mouse models causes increased embryonic or fetal death, supporting an essential role of autophagy in embryonic development. Central to the embryo’s survival is the formation of the placenta. This organ gains access to the maternal blood supply and facilitates the physiological exchange between the fetus and the maternal tissues. The placenta relies on a variety of specialized cells, called trophoblasts, and pathways that are essential for creating a sensing and physical barrier between the fetus and the mother.
Accordingly, autophagy is believed to play a critical role in placental development, monitoring all the challenges the embryo faces while adapting to the new maternal environment. The main goal of our research plan is to identify specific autophagy machinery required for placental development and maintenance during pregnancy.
When the placenta does not work properly, both mother and fetus display a variety of complex systemic disorders. An example is preeclampsia, where affected pregnant women develop hypertension and proteinuria along with systemic endothelial dysfunction during the second half of pregnancy and sometimes beyond delivery. Additionally, preeclampsia is sometimes associated with fetal growth restriction and stillbirth, conditions that critically impact the proper intra-uterine fetal development and have long-term adverse consequences later in the life of the child. The mechanisms that contribute to cardiovascular and renal disease following preeclampsia, unfortunately, are unclear. Therefore, the mission of the lab and our commitment to society is to understand such systemic pathologies, generate tools of diagnosis and treatment, and become a hub for collaborations with researchers from all disciplines within the interface of placental development and feto-maternal physiology.
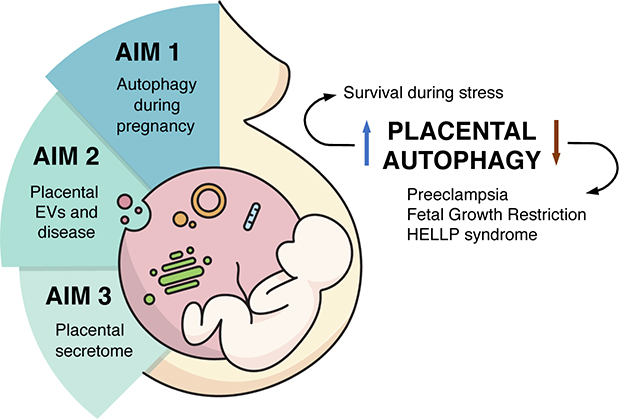
Major areas of research:
- Effects of the environment on placenta development and the role of the placenta in the Developmental Origin of Health and Disease (DOHaD).
- Development of new technologies to study placenta development in vitro.
- Placental-specific autophagy processes and the connection with the secretory pathway during development, function, and disease.
Current projects:
- Mechanisms of cargo-loading of placental extracellular vesicles (EVs).
- Molecular mechanisms of regulation of the secretion of hormones during placental development and pregnancy.
- Study of autophagy-specific placental cell functions in normal and pathological pregnancies.
Guardia earned his Ph.D. in Structural Biology from the University of Buenos Aires, Argentina in 2014, and then completed his postdoctoral training in Cell Biology at the Eunice Kennedy Shriver National Institute of Child Health and Human Development, under the supervision of Senior Investigator Juan S. Bonifacino, Ph.D., in 2021. During this time, Guardia conducted research on the mechanisms of lysosome movement and autophagy initiation using a multidisciplinary approach involving biochemistry, multi-"omics" tools, and several microscopy techniques, such as super-resolution fluorescence and cryo-electron microscopy. He became an NIH Stadtman Tenure-Track Investigator and a NIH Distinguished Scholar in December 2021 and joined NIEHS to establish his independent research program.
If you are interested in collaborating or joining the Placenta Group, please contact Guardia via email. Several trainee positions are available for postdoctoral fellows, postbaccalaureate students, and summer interns willing to train in the Research Triangle Park area of North Carolina.


