Environmental Cardiopulmonary Disease Group
Initial Findings
The Zeldin group was the first to:
- Clone CYP2J2, a human P450 enzyme that is active in the metabolism of arachidonic acid to epoxyeicosatrienoic acids (EETs) and is abundant in heart (Wu et al., J. Biol. Chem., 1996) (Figure 1)
- Show that EETs are endogenous constituents of cardiovascular tissues (Wu et al., J. Biol. Chem., 1996; Wu et al., J. Biol. Chem., 1997)
- Localize CYP2J2 expression to cardiac myocytes and endothelial cells (Wu et al., J. Biol. Chem., 1997; Node et al., Science, 1999)
- Demonstrate that soluble epoxide hydrolase (sEH) metabolizes biologically active EETs to less active dihydroxyeicosatrienoic acids (DHETs) (Zeldin et al, J. Biol. Chem., 1993)
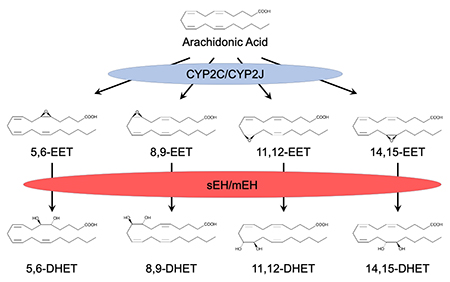
Figure 1. Metabolism of Arachidonic Acid by Cytochromes P450 and Epoxide Hydrolases. Arachidonic acid can be metabolized to epoxyeicosatrienoic acids (EETs) by a subset of cytochrome P450s which include members of the CYP2C and CYP2J subfamilies. EETs are hydrolyzed to less active dihydroxyeicosatrienioc acids (DHETs) by both soluble and microsomal epoxide hydrolase (sEH and mEH, respectively).
P450-Derived Eicosanoid Effects on Cardiovascular System Function
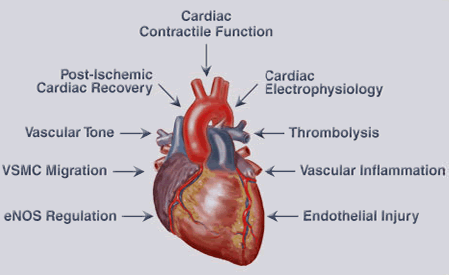
Figure 2. Biological Effects of EETs in the Cardiovascular System. EETs have many biological effects in the cardiovascular system. These effects include increasing cardiac contractile function, regulation of cardiac electrophysiology and protection against ischemia/reperfusion injury. EETs also induce vasodilation and thrombolysis, and attenuate vascular smooth muscle (VSMC) migration, vascular inflammation and endothelial injury.
Subsequent studies demonstrated that EETs improved heart contractile function following ischemia (Wu et al., J. Biol. Chem., 1997; Seubert et al., Circ. Res., 2004; Seubert et al., Circ. Res., 2006), inhibited cardiac L-type calcium channels (Chen et al., Mol. Pharmacol., 1999), activated ATP-sensitive potassium channels (Lu et al., J. Physiol., 2006). The group developed cardiomyocyte-specific CYP2J2 transgenic mice that have improved post-ischemic cardiac function and represent the first in vivo animal model for evaluating P450 functions in heart (Seubert et al., Circ. Res., 2004). The group showed that exposure of endothelial cells to hypoxia-reoxygenation decreased CYP2J2 expression and that maintenance of CYP2J2 protein levels attenuated hypoxia-reoxygenation-induced cell death (Yang et al., Mol. Pharmacol., 2001). Figure 2 summarizes the biological effects of EETs in the cardiovascular system.
The Zeldin group has developed transgenic mouse models with endothelial-specific expression of human CYP2C8 or CYP2J2 to investigate the role of endothelial EETs on cardiovascular function. Transgenic expression of either CYP2J2 or CYPC8 in endothelial cells increased EET generation enhanced vasodilatory responses, and attenuated vasoconstriction and hypertension in mice (Lee et al., FASEB J., 2010). In addition, endothelial CYP2J2 or CYP2C8 expression was protective against lung inflammation induced by intraperitoneal injection of bacterial lipopolysaccharide (LPS). Endothelial cell adhesion molecule, inflammatory cytokine production, and cellular infiltration was reduced in lungs from these mice, which suggests an important role for CYP epoxygenases in regulating host responses to infection (Deng et al., FASEB J., 2011).
The Zeldin group reported the detrimental effect of endothelial CYP2C8 expression on post-ischemic cardiac functional recovery. While increased EETs were cardioprotective following ischemia (Seubert et al., Circ. Res., 2004; Seubert et al., Circ. Res., 2006), endothelial CYP2C8 expression led to increased reactive oxygen species and linoleic acid-derived dihydroxyoctadecanoic acid (DiHOME) generation, which had vasoconstrictive and cardiodepressive effects in the post-ischemic heart (Edin et al., FASEB J., 2011). In collaboration with Craig Lee, Pharm.D, Ph.D. and Nancy Brown, M.D., the group found that EPHX2 (the gene that encodes sEH) polymorphisms correlate with vasodilatory responses in humans. This finding provides a functional link between human EPHX2 polymorphism and regulation of vascular tone (Lee et al., Hypertension., 2011).
The Zeldin group demonstrated that, despite slow in vitro kinetics, microsomal epoxide hydrolase (mEH) also significantly regulated EET levels. Disruption of both mEH and sEH synergistically increased EET levels and improved recovery after cardiac ischemia (Edin et al., J. Biol. Chem., 2018). In the heart, sEH was most strongly expressed in cardiomyoctyes where it regulated EET levels, kinase signaling and ion channel opening, and improved recovery of function after cardiac ischemia (Edin et al., J. Biol. Chem., 2023).
Together, these studies demonstrated that CYP2J2, CYP2C8, sEH and mEH regulate EET levels and play critical roles in cardiomyocytes and endothelial cells that control cardiac function following ischemia.
P450-Derived Eicosanoid Effects on Inflammation
In collaboration with James Liao, M.D., the group demonstrated that CYP2J2-derived eicosanoids decreased cytokine-induced endothelial adhesion molecule expression by a mechanism that involves inhibition of the transcription factor NF-kB (Node et al., Science, 1999).
Endothelial CYP2J2 or CYP2C8 expression increased EETs and protected against lung inflammation induced by intraperitoneal injection of bacterial LPS. Endothelial cell adhesion molecule, inflammatory cytokine production, and cellular infiltration was reduced in lungs from these mice, which suggests an important role for CYP epoxygenases in regulating host responses to infection (Deng et al., FASEB J., 2011). Interestingly, LPS induces a biphasic response in expression of many CYP2C and CYP2J isoforms in liver and intestine. Thus, LPS suppresses expression of CYP2C and CYP2J isoforms within 3-24 hours, but expression slowly returns to normal or above normal levels by 96 hours after LPS treatment. In contrast, there is a rise in epoxy fatty acid levels after LPS treatment that parallels PLA2-induced release of esterified fatty acids rather than LPS-induced suppression of CYP isoforms (Graves et al., FASEB J., 2020).
While mice with high EET levels have diminished inflammation in some disease models, global disruption of sEH exacerbated infection by S. pneumoniae. In response to bacterial wall components such as peptidogylcan, macrophages normally increase sEH to reduce anti-inflammatory EETs which allow efficient macrophage activation that is required for engulfment of bacteria. Disruption or inhibition of sEH in macrophages decreased inflammatory activation and bacterial engulfment which resulted in reduced S. pneumoniae clearance that exacerbated lung infection in mice (Li et al., JCI, 2021) (Figure 3).
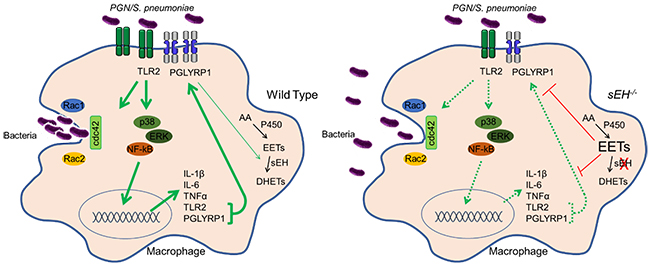
Figure 3. Induction of phagocytosis is impaired in sEH null macrophages. In response to S. pneumoniae, WT macrophages detect bacterial peptidoglycan (PGN) induce PGN receptor and cytokine expression and activate Rac and cdc42 to induce phagocytosis. In contrast, sEH null macrophages fail to efficiently induce PGN receptors and cytokines, have reduced Rac and cdc42 activation and diminished phagocytotic capacity.
Together, these studies demonstrated that CYP2J2, CYP2C8 and sEH regulate EET levels and activation of inflammatory circuits in response to bacterial infection.
P450-Derived Eicosanoid Effects on Renal System Function
Initial studies showed that P450-derived eicosanoids contribute substantially to integrated renal function (Figure 4). In 1999, the group cloned a new mouse P450 called CYP2J5 that is primarily expressed in the kidney, is active in the metabolism of arachidonic acid to EETs and localizes to proximal tubules and collecting ducts, sites where EETs affect renal fluid/electrolyte transport and mediate the actions of several hormones (Ma et al., J. Biol. Chem., 1999; Mol. Pharmacol., 2004). Interestingly, maximal CYP2J5 expression occurs during a critical time in postnatal renal development when abnormalities in renal function appear in animals that develop hypertension.
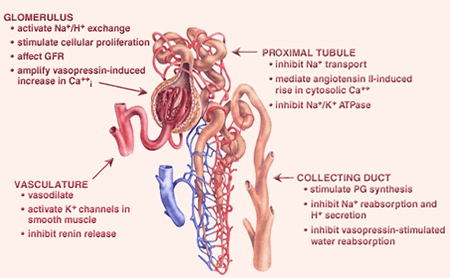
Figure 4. Biological Effects of EETs in the Kidney. EETs have many effects in the kidney. EETs activate potassium channels in smooth muscle cells to vasodilate renal blood vessels. EETs regulate sodium and hydrogen transport and vasopressin function from the glomerulus through the collecting ducts.
Further evidence to support the hypothesis that CYP2J products contribute to the development or maintenance of hypertension comes from two subsequent studies: one showing upregulation of a CYP2J immunoreactive protein and increased EET biosynthesis in kidneys of spontaneously hypertensive rats (Yu et al., Mol. Pharmacol., 2000) and another showing the chromosomal mapping of the Cyp2j gene cluster to an area that co-segregates with the hypertensive phenotype in Dahl salt-sensitive rats (Ma et al., Genomics, 1998).
The group generated Cyp2j5 knockout mice to investigate the role of CYP2J5 products on renal homeostasis. Female Cyp2j5-/- mice have increased blood pressure, enhanced tubular transport rates, and increased response to endogenous vasoconstrictors. This data suggests an important role for renal CYP epoxygenases in blood pressure regulation (Athirakul et al., FASEB J., 2008).
P450-Derived Eicosanoid Effects in Brain
The Zeldin group identified several functionally relevant EPHX2 polymorphic variants (Przybyla-Zawislak et al., Mol. Pharmacol, 2003) and demonstrated that one of these variants associates with cardiovascular disease and stroke in humans (Fornage et al., Hum. Mol. Genetics, 2005; Lee et al., Hum. Mol. Genetics, 2006). In collaboration with Daowen Wang, Ph.D., the group found that carriers of the EPHX2 R287Q polymorphism (reduced hydrolase activity, increased EETs) were protected against incidence of ischemic stroke in Han Chinese (Zhang et al., Pharmacogenet Genomics, 2008).
P450-Derived Eicosanoid Effects on Angiogenesis and Cancer
In collaboration with Daowen Wang, Ph.D., the Zeldin group has defined an important role for CYP epoxygenases and EETs in tumor growth and metastasis. CYP2J2 expression is abundant in human tumor tissues. Expression of CYP2J2 or treatment with synthetic EETs accelerates carcinoma cell proliferation, prevents apoptosis and enhances primary tumor grown in vivo (Jiang et al., Cancer Res, 2005). CYP2J2 is also upregulated in leukemia cells from peripheral blood and bone marrow in patients with malignant hematologic diseases (Chen et al., J Pharmacol Exp Ther, 2011). CYP epoxygenase expression or EET treatment also promotes tumor metastasis independent of effects on primary tumor growth. CYP2J2 expression induces cancer cell migration, invasion, adhesion, and colony formation in soft agar in vitro. Importantly, CYP epoxygenase expression enhances pro-metastatic proteins such as CD44 and leads to lung metastasis in vivo (Jiang et al., Cancer Res, 2007).
EETs also activate metalloproteinases which cleave and release heparin-bound EGF (HB-EGF). HB-EGF activates the EGF receptor to induce mitogenic signaling events and cancer cell proliferation (Cheng et al., Acta Pharmacol Sin, 2010). Interestingly, CYP epoxygenase inhibition or antisense to CYP2J2 blocks these effects. Together, these studies suggest the possibility that selective CYP2J2 inhibition may represent a novel strategy for treatment of cancer. In collaboration with Daniel Mansuy, Ph.D., and Pierre Lafite, Ph.D., we generated a 3D homology model of CYP2J2 and characterized the unusual regioselectivity and active site topology of CYP2J2 using terfenadine derivatives (Lafite et al., Bioorg Med Chem Lett, 2006). In addition a series of terfenadine and ebastine derivatives were found to be highly selective CYP2J2 inhibitors (Lafite et al., Arch Biochem Biophys, 2007).
In collaboration with Daowen Wang, Ph.D., we tested these and similar compounds for in vitro and in vivo anti-tumor activity. Each of the compounds decrease tumor cell proliferation, decrease tumor invasion and increase induction of apoptosis in vitro. In mice inoculated with MDA-MB-235 tumor cells, these terfenadine derivatives decreased tumor volume and increased median survival from 58 to 80 days, while showing no toxic effects in the mice (Chen et al., J Pharmacol Exp Ther, 2009). These studies provide proof-of-concept that terfenadine derivatives may represent novel chemotherapeutics for cancer in humans.
Previous reports revealed that CYP epoxygenases and EETs prevent tumor cell apoptosis while stimulating tumor cell growth, migration, and metastasis; however, the contribution of endothelial-derived EETs to tumor growth and metastasis was unknown. In collaboration with Dipak Panigrahy, M.D., we examined mice with increased endothelial expression of human P450 epoxygenases or with global sEH disruption. These ‘high-EET’ mice have increased endothelial proliferation and migration in vitro and increased primary tumor growth and metastasis in vivo. In contrast, mice with endothelial overexpression of human sEH have decreased EETs and exhibit reciprocal phenotypes. Pharmacological treatment with synthetic EETs or sEH inhibitors promoted tumor growth and metastasis, whereas treatment with the EET antagonist, 14,15-EEZE, reduced tumor growth and promoted survival (Panigrahy et al., J. Clin. Invest., 2012). Our subsequent studies with these mice showed that P450-derived EETs and sEH regulate angiogenesis in other disease models, including retinal vascularization, organ regeneration and wound healing (Panigrahy et al., PNAS, 2013). Together, these findings have clinical relevance as sEH inhibitors have been proposed as novel therapeutics for various diseases. In addition, our data suggests the potential use of EET antagonists as novel anti-neoplastic agents.
Additional studies revealed novel roles for P450-derived eicosanoids in endothelial function. In collaboration with Lois Smith, Ph.D, we determined that sEH and CYP2C enzymes regulate the aberrant retinal vascular development and detachment in oxygen-induced retinopathy, a model of retinal disease in premature infants (Shao et al, Arterioscler. Thromb. Vasc. Biol., 2014). In collaboration with Leonard Zon, M.D., we showed that EETs enhance hematopoietic stem and progenitor cell (HSPC) engraftment in a zebrafish bone marrow transplantation model (Li et al., Nature, 2015). Prior to these studies, our understanding of the role of CYPs, sEH and EETs in angiogenesis and related phenomena was limited to a handful of in vitro studies. CYP-derived EETs, acting through a common VEGF pathway involving PI3K activation, are now known to regulate angiogenesis in a wide variety of disease models in vivo.
Linoleic acid (LA)-rich diets have been promoted for their ability to reduce cholesterol levels and LA consumption in the U.S. has tripled over the past century. Interestingly, the rate of colorectal cancer has risen precipitously since the 1950s. In collaboration with Guodong Zhang, Ph.D., we discovered that LA-rich diet or LA-derived epoxyoctadecenoic acids (EpOMEs) promote colorectal tumor formation. Development of colorectal cancer was attenuated in mice treated with CYP2C inhibitors or with Cyp2c genetic disruption (Wang et al, Cancer Res 2019, Zhang et al, FASEB J., 2023).
Other Findings
The Zeldin group cloned and characterized the human CYP2J2 gene, and identified several functionally relevant CYP2J2 polymorphic variants (King et al., Mol. Pharmacol., 2002). One of these variants associates with reduced CYP2J2 expression/activity and is present at higher frequency in patients with cardiovascular disease versus normals (Spiecker et al., Circulation, 2004).
In addition to the CYP2J work, the group has cloned and characterized CYP2C8, the major human liver P450 arachidonic acid epoxygenase (Zeldin et al., Arch. Biochem. Biophys., 1995; Zeldin et al., Arch. Biochem. Biophys.,1996) and characterized several mouse CYP2C isoforms (Luo et al., Arch. Biochem. Biophys., 1998; Tsao et al., Mol. Pharmacol., 2000; Tsao et al., J. Pharmacol. Exp. Therap., 2001; DeLozier et al., J. Pharmacol. Exp. Therap., 2004; Wang et al., Mol. Pharmacol., 2004).
The group has identified two new P450 subfamilies, the CYP2Ns and the CYP2Ps, which relate phylogenetically to the CYP2Js. CYP2Ns and the CYP2Ps are abundant in extrahepatic tissues and are active in the metabolism of arachidonic acid to EETs (Oleksiak et al., J. Biol. Chem., 2000; Oleksiak et al., Arch. Biochem. Biophys., 2003).
The group was the first to document a role for sEH in the regio- and stereoselective metabolism of EETs to dihydroxyeicosatrienoic acids (DHETs) and to show that DHETs were endogenous constituents of liver and lung (Zeldin et al., Arch. Biochem. Biophys., 1995; Zeldin et al., Arch. Biochem. Biophys., 1996; Zeldin et al., J. Clin. Invest., 1995). In collaboration with Deanna Kroetz, Ph.D. of the University of California, San Francisco, the group’s data showed that sEH regulates EET hydrolysis and blood pressure in spontaneously hypertensive rats (Yu et al., Circ. Res., 2000).
Summary
Research in the Zeldin group has led to a better understanding of the role of cytochromes P450 in the metabolism of arachidonic acid to compounds which modulate fundamental biological processes in extrahepatic tissues, especially the heart, kidney, and vasculature. The opportunities for translational research in this area include development of novel therapeutics for blood pressure control, vascular inflammation, atherosclerosis, ischemic heart disease, and stroke. The identification of individuals who are at increased risk for these disorders due to P450 or sEH genetic variation may also lead to new approaches to disease prevention.


