Technology Transfer and Commercialization involves Science, Law, and Business activities that lead to the development of products beneficial to public health.
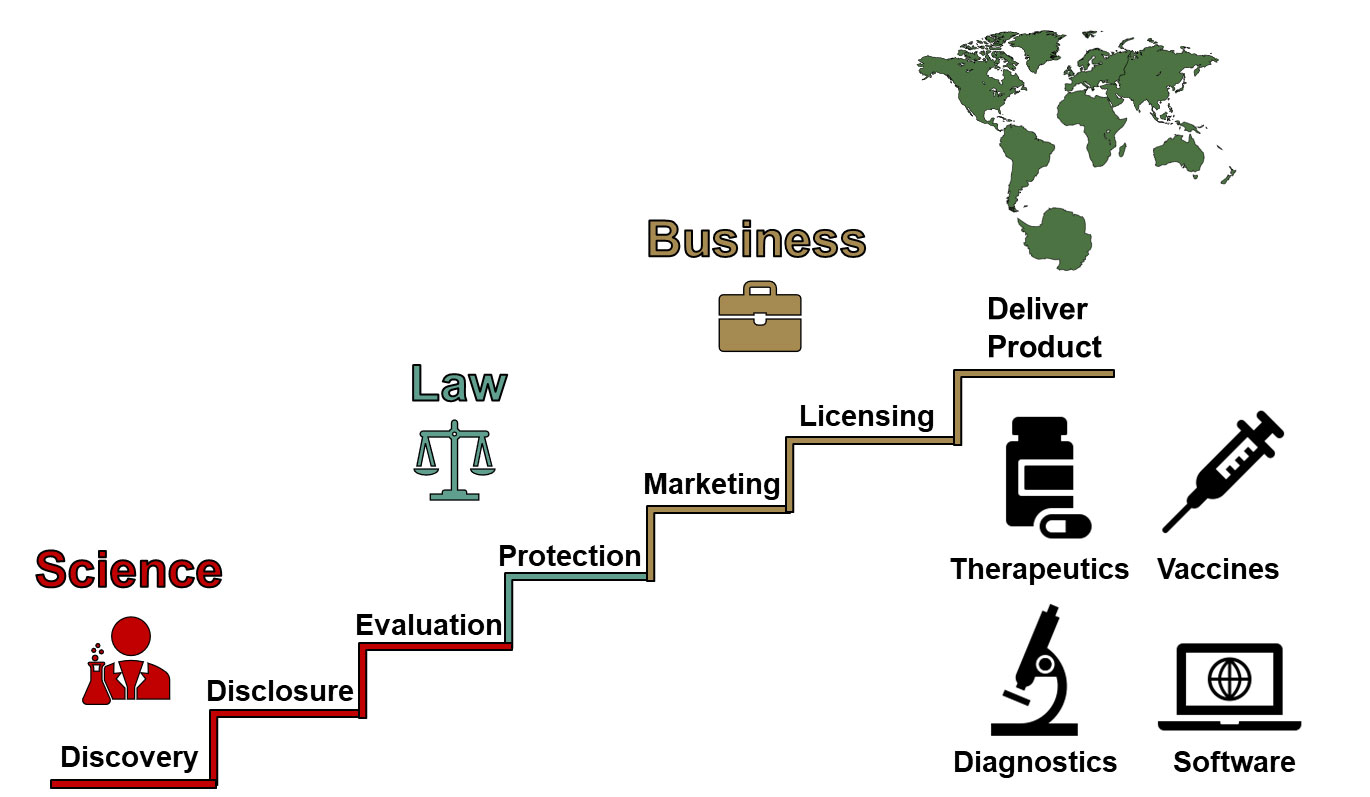
There are four overall stages in the technology transfer/commercialization process:
- Invention Disclosure
- Protection
- Marketing
- Licensing
Invention Disclosure
The first step in the invention review process is the formal disclosure of your invention. An Employee Invention Report (89KB) describes the technology and is used to evaluate the commercial potential of the technology. OTT investigates product applications, market potential, and strategizes patent prosecution.
OTT recommends disclosing invention information as soon as possible. Discussions with third parties or communication via emails and presentations may jeopardize patent protection. If not reported in a timely manner, your invention may not qualify for patent protection. It is critical that OTT is informed of all communications regarding your invention so that we can safeguard your technology and develop it to its fullest potential.
Why You Should Report Your Inventions As Early As Possible
Inventions must be reported as soon as possible, preferably before it is publicly disclosed in/on:
- Discussions with non-NIH personnel before execution of Confidential Disclosure Agreement
- Oral or poster presentations at conferences, symposia or seminars
- Job interviews, graduate student dissertations or theses
- Journal/magazine articles or website abstracts
- Social media/blog posts
Inventions must be reported before public disclosure because in the event of your invention being examined for a patent, it is found to be anticipated (or in other words, not novel) because of the presence of a prior public disclosure (like the ones listed above) that discusses the use or methods of your invention, the invention can be rejected for patent issuance by the Patent Office handling the case. The conditions for patentability (novelty and loss of right to patent, non-obvious subject matter) are outlined in 35 U.S.C. § 102 and 35 U.S.C. § 103.
Protection
After determining whether your invention will make a viable product that will have a commercial partner, the next step in the process is protection of your invention. Quite often a U.S. patent is utilized and provides protection for 20 years after the filing date, giving inventors an opportunity to fully develop and market products. This 20-year window allows inventors, commercial partners, and investors to recoup significant financial investment during the commercialization process.
NIEHS OTT relies on guidance from our service center in NHLBI and patent attorneys to navigate and draft the patent applications. Patent application packets contain many documents specified by the patent office, including an abstract describing your invention, specific claims for the invention, a background discussion of publicly available information, graphics or drawings of the invention, and a signed oath from the inventor. The OTT, inventors, and patent attorneys all work together to create the strongest application package possible, to give your invention the best chance of obtaining legal IP protection.
Marketing
NIEHS OTT will help identify potential commercial partners that have the resources and capacity to bring your technology to market. Often, you, the inventor, are the best resource for marketing through publications and collegial networking. We systematically market NIEHS technologies using a variety of marketing channels including our website, technology exchanges, and trade shows.
Licensing
NIEHS does not directly commercialize technologies and so inventions owned by the federal government are licensed to interested parties for commercial development. A license is an agreement where NIEHS grants another party permission to use the invention. After licensing, additional development for that technology to reach the market will be needed. Once a technology reaches the market, inventors are awarded a portion of royalties as set out in the licensing agreement. NIH employees can earn up to $150,000 a year in royalty income. FAQ on royalties for NIH inventors can be found at the Information for NIH Inventors website.
Copyright
Copyright protects original works of authorship including literary, dramatic, musical, and artistic either published or unpublished. It also protects various aspects of computer software. Authorship by a United States government employee that is done as part of the employee's official duties is a work of the United States Government. Copyright may not be established in the United States for works of government employees (17 U.S.C. § 105).
The 2024 NIH Public Access Policy requires Author Accepted Manuscripts accepted for publication in a journal, on or after July 1, 2025, to be submitted to PubMed Central upon acceptance for publication, for public availability without embargo upon the Official Date of Publication. NIH employees may not sign a journal's publishing agreement and instead required to use the " NIH Publishing Agreement & Manuscript Cover Sheet (183KB)."
For Peer-Reviewed Publications (must comply with Public Access Policy), the procedure posted on the NIH Public Access website provides guidance and procedures for NIH employees and a link to the NIH Publishing Agreement & Manuscript Cover Sheet (183KB). For Non-peer-reviewed publications such as books and book chapters, the procedure posted on the NIH Intramural Research Sourcebook website summarizes guidance and procedures for NIH authors of books, chapters and other non-peer reviewed materials. There is also a link to the Cover Sheet for use with non-peer-reviewed materials.
For help with a publisher who does not accept the coversheet, please contact OTT.
- NIH Form - Journal Articles (183KB)
- NIH Form - Books & Chapters (163KB)



