
Much of the work carried out by DTT is in support of the National Toxicology Program (NTP), an interagency partnership of the Food and Drug Administration, National Institute for Occupational Safety and Health, and NIEHS.
Molecular Toxicology & Genomics Group
-

-
Stephen S. Ferguson, Ph.D.
Chemist -
Tel 984-287-3128
[email protected] -
P.O. Box 12233Mail Drop E1-10Durham, NC 27709
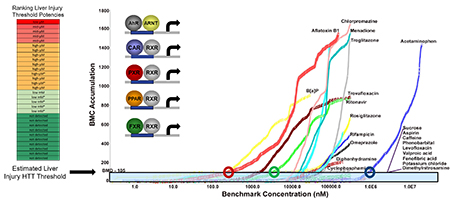
Stephen Ferguson is an Investigator within the Mechanistic Toxicology Branch of the Division of Translational Toxicology (DTT) leading multiple research initiatives to model and predict human responses to chemical exposures. His principal focus seeks to advance the development, qualification, and application of physiologically relevant in vitro screening models and microphysiological systems (MPS) that emulate integrated aspects of tissue functionality (e.g., liver, kidney) and biological response to xenobiotic exposures (e.g., per- and poly-fluorinated alkyl substances (PFAS), botanical mixtures, human therapeutics).
Prior to joining the DTT, Ferguson led the ADME-Tox R&D program of Life Technologies (now Thermo-Fisher) where he and his team developed advanced in vitro liver models and assay systems to predict human drug metabolism, transport, drug-drug interactions and hepatotoxicity. He received his BS and PhD degrees in chemistry and biotechnology from North Carolina State University, and currently serves as adjunct faculty to the Curriculum in Toxicology at the University of North Carolina at Chapel Hill.
Recent Publications:
- Rusyn I, Arzuaga X, Cattley RC, Corton JC, Ferguson SS, Godoy P, Guyton KZ, Kaplowitz N, Khetani SR, Roberts RA, Roth RA, Smith MT. 2021. Key characteristics of human hepatotoxicants as a basis for identification and characterization of the causes of liver toxicity. Hepatology 74(6):3486-3496. [Abstract Rusyn I, Arzuaga X, Cattley RC, Corton JC, Ferguson SS, Godoy P, Guyton KZ, Kaplowitz N, Khetani SR, Roberts RA, Roth RA, Smith MT. 2021. Key characteristics of human hepatotoxicants as a basis for identification and characterization of the causes of liver toxicity. Hepatology 74(6):3486-3496.]
- Buick JK, Williams A, Gagné R, Swartz CD, Recio L, Ferguson SS, Yauk CL. 2020. Flow cytometric micronucleus assay and TGx-DDI transcriptomic biomarker analysis of ten genotoxic and non-genotoxic chemicals in human HepaRG™ cells. Genes Environ; doi: 10.1186/s41021-019-0139-2 [Online 4 February 2020]. [Abstract Buick JK, Williams A, Gagné R, Swartz CD, Recio L, Ferguson SS, Yauk CL. 2020. Flow cytometric micronucleus assay and TGx-DDI transcriptomic biomarker analysis of ten genotoxic and non-genotoxic chemicals in human HepaRG™ cells. Genes Environ; doi: 10.1186/s41021-019-0139-2 [Online 4 February 2020].]
- Alaina N. Perkins, Nicole C. Deziel1, Salmaan H. Inayat-Hussain, Caroline H. Johnson, Stephen S. Ferguson, Abee L. Boyles, David C. Thompson and Vasilis Vasiliou. Evaluation of potential carcinogenicity of organic chemicals in synthetic turf crumb rubber. Environmental Research. 2018 169():163-172. [Abstract Alaina N. Perkins, Nicole C. Deziel1, Salmaan H. Inayat-Hussain, Caroline H. Johnson, Stephen S. Ferguson, Abee L. Boyles, David C. Thompson and Vasilis Vasiliou. Evaluation of potential carcinogenicity of organic chemicals in synthetic turf crumb rubber. Environmental Research. 2018 169():163-172.]
- Jackson, JP, Linhao, L, Deibert, E, Wang, H, Ferguson, SS. 2016. Contextualizing Hepatocyte Functionality of Cryopreserved HepaRG® Cell Cultures. Drug metabolism and disposition: the biological fate of chemicals. 44(9):1463-1479. [Abstract Jackson, JP, Linhao, L, Deibert, E, Wang, H, Ferguson, SS. 2016. Contextualizing Hepatocyte Functionality of Cryopreserved HepaRG® Cell Cultures. Drug metabolism and disposition: the biological fate of chemicals. 44(9):1463-1479.]
- Wetmore BA, Wambaugh JF, Allen B, Ferguson SS, Sochaski MA, Setzer RW, Houck KA, Strope CL, Cantwell K, Judson RS, LeCluyse E, Clewell HJ, Thomas RS, Andersen ME. Toxicol Sci. 2015 Nov;148(1):121-36. doi: 10.1093/toxsci/kfv171. Epub 2015 Aug 6. [Abstract Wetmore BA, Wambaugh JF, Allen B, Ferguson SS, Sochaski MA, Setzer RW, Houck KA, Strope CL, Cantwell K, Judson RS, LeCluyse E, Clewell HJ, Thomas RS, Andersen ME. Toxicol Sci. 2015 Nov;148(1):121-36. doi: 10.1093/toxsci/kfv171. Epub 2015 Aug 6.]
- Kirman CR, Aylward LL, Wetmore BA, Thomas RS, Sochaski M, Ferguson SS, Csiszar SA, and Olivier J. Applied In Vitro Toxicology. 2015 Jun; 1(2):140-146. doi:10.1089/aivt.2014.0008. Epub 2015 Feb12. [Abstract Kirman CR, Aylward LL, Wetmore BA, Thomas RS, Sochaski M, Ferguson SS, Csiszar SA, and Olivier J. Applied In Vitro Toxicology. 2015 Jun; 1(2):140-146. doi:10.1089/aivt.2014.0008. Epub 2015 Feb12.]
- Bonzo JA, Rose K, Freeman K, Deibert E, Amaral K, Ferguson SS, Anderson ME, Witek, RP, LeCluyse EL. Applied In Vitro Toxicology. 2015 Mar; 1(1):45-54. doi:10.1089/aivt.2014.0004. Epub 2015 Feb11. [Abstract Bonzo JA, Rose K, Freeman K, Deibert E, Amaral K, Ferguson SS, Anderson ME, Witek, RP, LeCluyse EL. Applied In Vitro Toxicology. 2015 Mar; 1(1):45-54. doi:10.1089/aivt.2014.0004. Epub 2015 Feb11.]
- Richardson VM, Ferguson SS, Sey YM, Devito MJ. Xenobiotica. 2014 May;44(5):391-403. doi:10.3109/00498254.2013.847990. Epub 2013 Oct 31. [Abstract Richardson VM, Ferguson SS, Sey YM, Devito MJ. Xenobiotica. 2014 May;44(5):391-403. doi:10.3109/00498254.2013.847990. Epub 2013 Oct 31.]
- Elcombe CR, Peffer RC, Wolf DC, Bailey J, Bars R, Bell D, Cattley RC, Ferguson SS, Geter D, Goetz A, Goodman JI, Hester S, Jacobs A, Omiecinski CJ, Schoeny R, Xie W, Lake BG. Crit Rev Toxicol. Jan; 44(1):64-82. doi: 10.3109/10408444.2013.835786. Epub 2013 Nov 4. Review. [Abstract Elcombe CR, Peffer RC, Wolf DC, Bailey J, Bars R, Bell D, Cattley RC, Ferguson SS, Geter D, Goetz A, Goodman JI, Hester S, Jacobs A, Omiecinski CJ, Schoeny R, Xie W, Lake BG. Crit Rev Toxicol. Jan; 44(1):64-82. doi: 10.3109/10408444.2013.835786. Epub 2013 Nov 4. Review.]
- Wetmore BA, Wambaugh JF, Ferguson SS, Li L, Clewell HJ 3rd, Judson RS, Freeman K, Bao W, Sochaski MA, Chu TM, Black MB, Healy E, Allen B, Andersen ME, Wolfinger RD, Thomas RS. 2013. Relative impact of incorporating pharmacokinetics on predicting in vivo hazard and mode of action from high-throughput in vitro toxicity assays. Toxicol Sci. 132(2):327-346. [Abstract Wetmore BA, Wambaugh JF, Ferguson SS, Li L, Clewell HJ 3rd, Judson RS, Freeman K, Bao W, Sochaski MA, Chu TM, Black MB, Healy E, Allen B, Andersen ME, Wolfinger RD, Thomas RS. 2013. Relative impact of incorporating pharmacokinetics on predicting in vivo hazard and mode of action from high-throughput in vitro toxicity assays. Toxicol Sci. 132(2):327-346.]
- Wang D, Li L, Yang H, Ferguson SS, Baer MR, Gartenhaus RB, Wang H. 2013. The constitutive androstane receptor is a novel therapeutic target facilitating cyclophosphamide-based treatment of hematopoietic malignancies. Blood. 121(2):329-38. [Abstract Wang D, Li L, Yang H, Ferguson SS, Baer MR, Gartenhaus RB, Wang H. 2013. The constitutive androstane receptor is a novel therapeutic target facilitating cyclophosphamide-based treatment of hematopoietic malignancies. Blood. 121(2):329-38.]
- Lynch C, Pan Y, Li L, Ferguson SS, Xia M, Swaan PW, Wang H. 2013. Identification of novel activators of constitutive androstane receptor from FDA-approved drugs by integrated computational and biological approaches. Pharm Res. 30(2):489-501. [Abstract Lynch C, Pan Y, Li L, Ferguson SS, Xia M, Swaan PW, Wang H. 2013. Identification of novel activators of constitutive androstane receptor from FDA-approved drugs by integrated computational and biological approaches. Pharm Res. 30(2):489-501.]
- Smith CM, Nolan CK, Edwards MA, Hatfield JB, Stewart TW, Ferguson SS, Lecluyse EL, Sahi J. J. 2012. A comprehensive evaluation of metabolic activity and intrinsic clearance in suspensions and monolayer cultures of cryopreserved primary human hepatocytes. Pharm Sci. 101(10):3989-4002. [Abstract Smith CM, Nolan CK, Edwards MA, Hatfield JB, Stewart TW, Ferguson SS, Lecluyse EL, Sahi J. J. 2012. A comprehensive evaluation of metabolic activity and intrinsic clearance in suspensions and monolayer cultures of cryopreserved primary human hepatocytes. Pharm Sci. 101(10):3989-4002.]
- Zhang SY, Surapureddi S, Coulter S, Ferguson SS, Goldstein JA. 2012. Human CYP2C8 is post-transcriptionally regulated by microRNAs 103 and 107 in human liver. Mol Pharmacol. 2012 Jun 20 82(3):529-40. [Abstract Zhang SY, Surapureddi S, Coulter S, Ferguson SS, Goldstein JA. 2012. Human CYP2C8 is post-transcriptionally regulated by microRNAs 103 and 107 in human liver. Mol Pharmacol. 2012 Jun 20 82(3):529-40.]
- Black MB, Budinsky RA, Dombkowski A, Cukovic D, LeCluyse EL, Ferguson SS, Thomas RS, Rowlands JC. 2012.Cross-species comparisons of transcriptomic alterations in human and rat primary hepatocytes exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin. Toxicol Sci. 127(1):199-215. [Abstract Black MB, Budinsky RA, Dombkowski A, Cukovic D, LeCluyse EL, Ferguson SS, Thomas RS, Rowlands JC. 2012.Cross-species comparisons of transcriptomic alterations in human and rat primary hepatocytes exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin. Toxicol Sci. 127(1):199-215.]
- Wetmore BA, Wambaugh JF, Ferguson SS, Sochaski MA, Rotroff DM, Freeman K, Clewell HJ 3rd, Dix DJ, Andersen ME, Houck KA, Allen B, Judson RS, Singh R, Kavlock RJ, Richard AM, Thomas RS. 2012. Integration of dosimetry, exposure and high-throughput screening data in chemical toxicity assessment. Toxicol Sci. 125(1):157-74. [Abstract Wetmore BA, Wambaugh JF, Ferguson SS, Sochaski MA, Rotroff DM, Freeman K, Clewell HJ 3rd, Dix DJ, Andersen ME, Houck KA, Allen B, Judson RS, Singh R, Kavlock RJ, Richard AM, Thomas RS. 2012. Integration of dosimetry, exposure and high-throughput screening data in chemical toxicity assessment. Toxicol Sci. 125(1):157-74.]
- Wang D, Li L, Fuhrman J, Ferguson S, Wang H. 2011.The role of constitutive androstane receptor in oxazaphosphorine-mediated induction of drug-metabolizing enzymes in human hepatocytes. Pharm Res. 28(8):2034-44. [Abstract Wang D, Li L, Fuhrman J, Ferguson S, Wang H. 2011.The role of constitutive androstane receptor in oxazaphosphorine-mediated induction of drug-metabolizing enzymes in human hepatocytes. Pharm Res. 28(8):2034-44.]
- Rana, R., S. Surapureddi, W. Kam, S. Ferguson and J. A. Goldstein (2011). "Med25 is required for RNA polymerase II recruitment to specific promoters, thus regulating xenobiotic and lipid metabolism in human liver." Molecular and cellular biology 31(3): 466-481.
- Li Y, Zhou D, Ferguson SS, Dorff P, Simpson TR, Grimm SW. 2010. In vitro assessment of metabolic drug–drug interaction potential of AZD2624, neurokinin-3 receptor antagonist, through cytochrome P(450) enzyme identification, inhibition, and induction studies. Xenobiotica. 40(11):721-9. [Abstract Li Y, Zhou D, Ferguson SS, Dorff P, Simpson TR, Grimm SW. 2010. In vitro assessment of metabolic drug–drug interaction potential of AZD2624, neurokinin-3 receptor antagonist, through cytochrome P(450) enzyme identification, inhibition, and induction studies. Xenobiotica. 40(11):721-9.]
- Martinez SM, Bradford BU, Soldatow VY, Kosyk O, Sandot A, Witek R, Kaiser R, Stewart T, Amaral K, Freeman K, Black C, LeCluyse EL, Ferguson SS, Rusyn I. 2010. Evaluation of an in vitro toxicogenetic mouse model for hepatotoxicity. Toxicol Appl Pharmacol. 249(3):208-216. [Abstract Martinez SM, Bradford BU, Soldatow VY, Kosyk O, Sandot A, Witek R, Kaiser R, Stewart T, Amaral K, Freeman K, Black C, LeCluyse EL, Ferguson SS, Rusyn I. 2010. Evaluation of an in vitro toxicogenetic mouse model for hepatotoxicity. Toxicol Appl Pharmacol. 249(3):208-216.]
- Li L, Hassan HE, Tolson AH, Ferguson SS, Eddington ND, Wang H. 2010. Differential activation of pregnane X receptor and constitutive androstane receptor by buprenorphine in primary human hepatocytes and hepG2 cells. J Pharmacol Exp Ther. 335(3):562-571. [Abstract Li L, Hassan HE, Tolson AH, Ferguson SS, Eddington ND, Wang H. 2010. Differential activation of pregnane X receptor and constitutive androstane receptor by buprenorphine in primary human hepatocytes and hepG2 cells. J Pharmacol Exp Ther. 335(3):562-571.]
- Budinsky RA, LeCluyse EL, Ferguson SS, Rowlands JC, Simon T. 2010. Human and rat primary hepatocyte CYP1A1 and 1A2 induction with 2,3,7,8-tetrachlorodibenzo-p-dioxin, 2,3,7,8-tetrachlorodibenzofuran, and 2,3,4,7,8-pentachlorodibenzofuran. Toxicol Sci. 118(1):224-235. [Abstract Budinsky RA, LeCluyse EL, Ferguson SS, Rowlands JC, Simon T. 2010. Human and rat primary hepatocyte CYP1A1 and 1A2 induction with 2,3,7,8-tetrachlorodibenzo-p-dioxin, 2,3,7,8-tetrachlorodibenzofuran, and 2,3,4,7,8-pentachlorodibenzofuran. Toxicol Sci. 118(1):224-235.]
- Rotroff DM, Wetmore BA, Dix DJ, Ferguson SS, Clewell HJ, Houck KA, Lecluyse EL, Andersen ME, Judson RS, Smith CM, Sochaski MA, Kavlock RJ, Boellmann F, Martin MT, Reif DM, Wambaugh JF, Thomas RS. 2010. Incorporating human dosimetry and exposure into high-throughput in vitro toxicity screening. Toxicol Sci. 117(2):348-358. [Abstract Rotroff DM, Wetmore BA, Dix DJ, Ferguson SS, Clewell HJ, Houck KA, Lecluyse EL, Andersen ME, Judson RS, Smith CM, Sochaski MA, Kavlock RJ, Boellmann F, Martin MT, Reif DM, Wambaugh JF, Thomas RS. 2010. Incorporating human dosimetry and exposure into high-throughput in vitro toxicity screening. Toxicol Sci. 117(2):348-358.]
- Li, H, Ferguson, S.S., Wang, H. 2010. Synergistically enhanced CYP2B6 inducibility between a polymorphic mutation in CYP2B6 promoter and pregnane X receptor activation. 2010. Mol Pharmacol. 78(4):704-713. [Abstract Li, H, Ferguson, S.S., Wang, H. 2010. Synergistically enhanced CYP2B6 inducibility between a polymorphic mutation in CYP2B6 promoter and pregnane X receptor activation. 2010. Mol Pharmacol. 78(4):704-713.]
- Rotroff DM, Beam AL, Dix DJ, , Farmer A, Freeman KM, Houck KA, Judson RS, LeCluyseEL, Martin MT, Reif DM, Ferguson SS. 2010. Xenobiotic metabolizing enzyme and transporter gene expression in primary cultures of human hepatocytes modulated by ToxCast chemicals. J. Toxicol.Env. Health B Crit Rev. 13(2-4):329-346. [Abstract Rotroff DM, Beam AL, Dix DJ, , Farmer A, Freeman KM, Houck KA, Judson RS, LeCluyseEL, Martin MT, Reif DM, Ferguson SS. 2010. Xenobiotic metabolizing enzyme and transporter gene expression in primary cultures of human hepatocytes modulated by ToxCast chemicals. J. Toxicol.Env. Health B Crit Rev. 13(2-4):329-346.]
- Rana R, Chen Y, Ferguson S, Kissling GE, Surapureddi S, Goldstein JA. Hepatocyte nuclear factor 4α regulates rifampicin-mediated induction of CYP2C genes in primary cultures of human hepatocytes. Drug Metabolism and Disposition 2010; 38:591-599. [Abstract Rana R, Chen Y, Ferguson S, Kissling GE, Surapureddi S, Goldstein JA. Hepatocyte nuclear factor 4α regulates rifampicin-mediated induction of CYP2C genes in primary cultures of human hepatocytes. Drug Metabolism and Disposition 2010; 38:591-599.]


