Introduction
Parkinson's Disease

Parkinson's Disease
NIEHS is one of the lead research agencies studying environmental links to Parkinson’s disease. The institute works closely with other research programs within the National Institutes of Health (NIH), as well as partners and scientists across the country, to look at every aspect of this disease.
What Is Parkinson's Disease?
Parkinson's disease is neurodegenerative, the second most common disorder of this type after Alzheimer's disease.
Within your body, nerves transmit information to and from the brain or spinal cord, which affects muscles and organs. Neurodegeneration means that your nerves are not functioning normally. Parkinson’s disease is a progressive and unending movement disorder.
How Many People Are Affected by Parkinson's Disease?
Parkinson's may be the world's fastest growing brain disease. In 2016, scientists estimated about 6 million individuals had Parkinson's disease globally, compared with 2.5 million in 1990.
Parkinson's strikes people of all races, ethnic groups, nationalities, and income levels. Well-known people with the disease include actors Michael J. Fox and Alan Alda, singer Linda Ronstadt, and civil rights activist Jesse Jackson.
What Are the Symptoms of Parkinson's Disease?
Common motor symptoms include:
- Tremors or shaking in hands, arms, legs, jaw, and face
- Rigidity or stiffness of limbs and trunk
- Slowness of movement
- Difficulties with balance, speech, and coordination
There are also non-motor symptoms which may develop years before the onset of motor problems, which may include:
- Constipation
- Cognitive impairment
- Depression
- Fatigue
- Poor sense of smell
- REM sleep behavior disorder (dream-enacting behavior)
Disease warning signs, such as tremors, loss of grip strength, and gait disruption, begin gradually and typically worsen over time. Symptoms and their progression may vary. Parkinson's disease cannot be cured, but treatments and drugs can help control it.
What Causes Parkinson's Disease?
The exact cause of Parkinson's disease is unknown. Most people diagnosed with Parkinson’s have a late-onset form of the disease, which does not have a clear genetic cause. Early-onset Parkinson's, defined as beginning before age 50, accounts for 2% to 10% of cases. This form may have a genetic cause.
Parkinson’s disease likely results from both genetic and environmental factors, and interactions among these factors. A full understanding of Parkinson's risk requires integrated efforts to study both genetic and environmental factors.
- Environmental toxicants could cause stress and damage to cells, triggering inflammation in the brainstem.
- These exposures could impair mitochondria, the structures within cells responsible for producing energy, and accelerate aging in the brain.
- Toxicant exposure may also trigger widespread accumulation of a misfolded version of protein called alpha-synuclein, which is toxic to nerve cells and a hallmark of Parkinson’s disease.
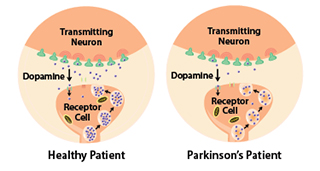
Studies have shown that the symptoms of Parkinson's usually appear when 50% or more of the dopamine neurons in the midbrain are lost. It progresses slowly as small clusters of neurons in the middle of the brain die. The gradual loss of these neurons results in reduction of a chemical called dopamine, which is responsible for transmitting messages to parts of the brain that coordinate muscle movement.
Decades of research suggest several biological processes could drive the nerve cell death seen in Parkinson’s disease.
Parkinson’s disease destroys nerve cells in the brain, yet some researchers think it may start in the gut. Abnormal clumps of misfolded alpha-synuclein proteins have been found in the intestines of people with Parkinson’s disease. These clumps may cause nearby proteins to misfold and clump as well, triggering a chain reaction that travels all the way up the vagus nerve that connects directly to the brain.
There is a critical need for research investment that leads to better understanding of Parkinson’s disease. Identifying environmental exposures related to the onset of Parkinson’s may lead to new prevention and intervention strategies.
What is NIEHS Doing?
NIEHS supports diverse research, involving experts from many disciplines, to uncover what may cause or help prevent Parkinson’s disease. Varied methods are important because no one can predict which paths of study will provide major breakthroughs. Basic research on Parkinson's will continue to help us advance our understanding of the disease. Highlights from NIEHS research are described below, grouped by environmental factors that may affect Parkinson’s and by research approaches.
Pesticides

Pesticide exposure has consistently been associated with the onset of Parkinson's disease.
- A 17-year long, NIEHS-funded study of the links among Parkinson’s Disease, Environment, and Genes shows that some pesticides, including paraquat, maneb, ziram, benomyl, and several organophosphate pesticides, including diazinon and chlorpyrifos, contribute to Parkinson’s disease onset and progression.
- People with Parkinson’s disease who are exposed to a high level of 10 different active ingredients in agricultural pesticides may see their motor and non-motor symptoms progress faster compared with those who are not, a 2022 study found.
- Other NIEHS research found that people who occupationally used two pesticides, rotenone or paraquat, developed Parkinson’s disease 2.5 times more often than nonusers.1
- In addition, people exposed to pesticides in the home or garden may face a greater chance of developing Parkinson’s disease.2
- Pesticides may directly or indirectly disrupt the biological pathways that normally protect dopaminergic neurons, the brain cells selectively attacked by the disease.3
- Some pesticides, like rotenone, can directly block the function of mitochondria, the structures that create energy to run the cell. NIEHS researchers showed in mice that disrupting mitochondria through exposure to rotenone early in development changed the epigenome – the chemical tags that turn genes on and off – in ways that persisted throughout life.4
- Other pesticides, like paraquat, have been found to increase production of free radicals that can damage cells.
Some people are more vulnerable to the harmful effects of pesticides because of their age or genetic makeup.
- Many studies identified genetic variations that provide insight into why certain people appear to be at higher risk of developing Parkinson’s.
- Using data from the NIEHS-conducted Agricultural Health Study, researchers found that Parkinson's risk from paraquat use was particularly high in people with a particular variant of a gene known as GSTT1.5
- Similarly, other research has indicated that people with lower levels of the PON1 gene, which is important for the metabolism of organophosphate pesticides, showed faster progression of the disease.6
Further research into links between preventable exposures and Parkinson’s disease, as well as preventative therapies, could help reduce the incidence of the disease. For example, using protective gloves and other hygiene practices reduced the risk of Parkinson’s disease among farmers using paraquat, permethrin, and trifluralin.7
Trichloroethylene (TCE)
TCE is a chemical compound used in industrial degreasing, dry cleaning, and some household cleaning products. It can be released into the environment from industrial sites, contaminating air, water, and soil.
The National Toxicology Program states TCE is known to be a human carcinogen based on sufficient evidence of carcinogenicity from studies in humans. It has been linked to kidney, liver, and blood cancers.
NIEHS-funded researchers show exposure to TCE may affect protein kinase LRRK2, a gene that is active in the brain and other body tissues, and elevate the risk of Parkinson's.
Head Injuries
Numerous studies over the years suggest that head injuries could contribute to Parkinson's disease through the death of dopaminergic neurons. Head injuries can be particularly damaging for people exposed to pesticides such as paraquat8 as well as those who carry a genetic risk factor in the alpha-synuclein gene.9 Individuals with head injuries who lived or worked near areas where paraquat had been applied were several times more likely to develop Parkinson’s disease.
Air Pollution
Air pollution is a mixture of hazardous substances from both human-made and natural sources. The harmful effects of air pollution on the heart and lungs are well-documented, although effects on the brain are less understood. Recent studies suggest that air pollution might act on the biological pathways that are involved in Parkinson’s disease. For example, one study found that exposure to high air pollution levels caused neuroinflammation, an altered brain immune response, and an accumulation of toxic alpha-synuclein deposits starting in childhood.10
Using state-of-the-art geospatial analytical techniques, NIEHS-funded researchers were able to confirm a strong association between cases of Parkinson’s disease and fine particulate matter (known as PM2.5) across the U.S. In the study, regions of the country with a high rate of Parkinson’s disease were associated generally with higher levels of PM2.5, of which sources include motor vehicles, wildfires, and power plants.
Diet and Lifestyle

Diet and lifestyle factors may affect the development and progression of Parkinson's disease. Understanding the role that these factors could play in the disease could inform future prevention and treatment.
Caffeine. Caffeine may reduce the chance of developing the disease. NIEHS researchers looked at data from a large sample of older Americans, and found that higher caffeine intake was associated with lower risk of Parkinson's in both men and women.11 A separate study found that Parkinson’s disease patients who were lifelong coffee drinkers had slower progression, less cognitive decline, and reduced mortality.12 Animal studies have also shown that caffeine can protect the brain's dopaminergic neurons.
Cooking meat. Heterocyclic amines (HCAs) are chemicals that are produced during high temperature meat cooking. These compounds have been investigated for their role in cancer, but not as much for their effect on neurodegenerative diseases. Working in animal models, NIEHS grantees showed that a variety of different HCAs were selectively toxic to the dopaminergic neurons affected in Parkinson’s disease. The findings are important because HCAs are a commonly consumed dietary component. 13
Vitamin D. Several studies suggest vitamin D deficiency may be involved in the development of Parkinson's.14 Vitamin D, obtained through food or sunlight, plays an important role in regulating the normal function of brain cells, as well as in maintaining good balance and muscle strength. NIEHS-funded scientists at the UCLA School of Public Health found genetic variants that affect the body’s ability to respond to vitamin D may influence a person’s susceptibility to cognitive decline in Parkinson’s disease. Additional studies are needed to further clarify this association.15

Exercise. Regular exercise can improve a person’s quality of life and can influence many chronic diseases, including cardiovascular disease and cancer. Several studies suggest that those benefits may extend to protecting against Parkinson's disease. In older U.S. adults, higher levels of moderate to vigorous physical activity during middle age were associated with lower risk of Parkinson's.16 Exercise may also benefit patients with the disease, by improving balance and reducing depression. For example, some work has suggested that tai chi training in patients with mild to moderate Parkinson's improved balance and reduced falls. A recent study showed that a history of competitive sports and more lifelong physical exercise also predicted slower motor and cognitive decline in patients with Parkinson’s disease.17
Heavy metals and the workplace. Welders exposed to the heavy metal manganese may develop symptoms associated with Parkinson’s disease, including slowness of movement in the arms and hands, speech problems, and reduced facial expressions. These symptoms may get worse the more workers are exposed, even when the concentrations of manganese are below the regulatory limit.18 NIEHS grantees discovered how manganese exposure can lead to aggregation and spread of toxic clumps of alpha-synuclein proteins.19 This study provides new information about the biological processes that link manganese exposure and the onset of Parkinson’s-like symptoms.
Predicting Disease
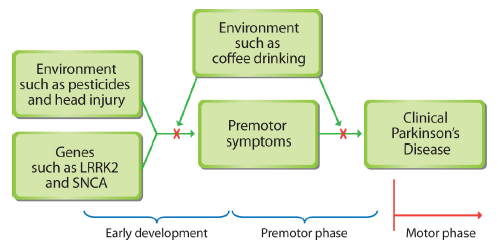
Premotor symptoms of Parkinson’s disease may occur years before some muscular problems surface. By thinking of Parkinson's as a systemic illness that takes decades to develop, researchers may better understand the causes of the disease and its progression. Some early warning indicators include premotor symptoms, including constipation, loss of smell, fatigue, and mood or anxiety disorders.
NIEHS-funded researchers developed a mathematical algorithm that could provide an early indicator to physicians that patients may need to be evaluated for the disease. 20 The predictive model relies on demographic data, as well as medical tests and diagnoses available in Medicare claims data, to identify people with a high probability of being diagnosed with Parkinson’s disease. This method may also be used to identify other factors, such as environmental hazards, that may be associated with a higher or lower risk of the disease.
Other work by NIEHS-funded scientists identified a chemical signature, or biomarker, in blood that predicts the rapid progression of the disease’s motor progression. 21 The scientists also identified biomarkers that suggest the immune system of Parkinson’s disease patients ages faster. The findings represent an important step forward in understanding how the illness evolves over time.
Understanding Disease Progression
Over the past few decades, the development and use of animal models have provided valuable insight into the symptoms of Parkinson's and how the disease progresses. 22 Learning what causes the selective loss of dopamine neurons may inform development of new therapies that slow or reverse disease progression.
Other researchers are using nematodes and zebrafish to test the role that some chemicals may play in the brain, and to better understand how neurodegeneration occurs.
Brain imaging techniques and genome-wide association studies provide additional insight into the molecular causes of Parkinson's.
Grant-funded Research at NIEHS
Beyond the above-mentioned research findings, NIEHS is funding more than 45 grant projects related to Parkinson's disease.
In-house Research at NIEHS
At NIEHS, multidisciplinary teams of distinguished researchers work in our labs to understand the role of the environment, genes, and gene-environment interactions in Parkinson’s disease.
- The Ion Channel Physiology Group studies the structure, function and regulation of the Cys-loop ligand-gated ion channel superfamily, with a particular focus on the nicotinic acetylcholine receptor channels and their role in neurological disorders.
- The Neuropharmacology Group has several projects to explain mechanisms involved in the pathogenesis of Parkinson's disease and to develop novel therapeutic agents that target these mechanisms as a means to halt disease progress.
-
Tanner CM, Kamel F, Ross GW, Hoppin JA, Goldman SM, Korell M, Marras C, Bhudhikanok GS, Kasten M, Chade AR, Comyns K, Richards MB, Meng C, Priestley B, Fernandez HH, Cambi F, Umbach DM, Blair A, Sandler DP, Langston JW. 2011. Rotenone, paraquat, and Parkinson's disease. Environ Health Perspect 119(6):866-872. [Abstract Tanner CM, Kamel F, Ross GW, Hoppin JA, Goldman SM, Korell M, Marras C, Bhudhikanok GS, Kasten M, Chade AR, Comyns K, Richards MB, Meng C, Priestley B, Fernandez HH, Cambi F, Umbach DM, Blair A, Sandler DP, Langston JW. 2011. Rotenone, paraquat, and Parkinson's disease. Environ Health Perspect 119(6):866-872.]
-
Kochmanski J, VanOeveren SE, Patterson JR, Bernstein AI. 2019. Developmental dieldrin exposure alters DNA methylation at genes related to dopaminergic neuron development and Parkinson's disease in mouse midbrain. Toxicol Sci. 169(2):593-607. [Abstract Kochmanski J, VanOeveren SE, Patterson JR, Bernstein AI. 2019. Developmental dieldrin exposure alters DNA methylation at genes related to dopaminergic neuron development and Parkinson's disease in mouse midbrain. Toxicol Sci. 169(2):593-607.]
-
Lozoya OA, Xu F, Grenet D, Wang T, Grimm SA, Godfrey V, Waidyanatha S, Woychik RP, Santos JH. 2020. Single nucleotide resolution analysis reveals pervasive, long-lasting DNA methylation changes by developmental exposure to a mitochondrial toxicant. Cell Rep. 32(11):108131. [Abstract Lozoya OA, Xu F, Grenet D, Wang T, Grimm SA, Godfrey V, Waidyanatha S, Woychik RP, Santos JH. 2020. Single nucleotide resolution analysis reveals pervasive, long-lasting DNA methylation changes by developmental exposure to a mitochondrial toxicant. Cell Rep. 32(11):108131.]
-
Goldman SM, Kamel F, Ross GW, Bhudhikanok GS, Hoppin JA, Korell M, Marras C, Meng C, Umbach DM, Kasten M, Chade AR, Comyns K, Richards MB, Sandler DP, Blair A, Langston JW, Tanner CM. 2012. Genetic modification of the association of paraquat and Parkinson's disease. Mov Disord. (13):1652-8. [Abstract Goldman SM, Kamel F, Ross GW, Bhudhikanok GS, Hoppin JA, Korell M, Marras C, Meng C, Umbach DM, Kasten M, Chade AR, Comyns K, Richards MB, Sandler DP, Blair A, Langston JW, Tanner CM. 2012. Genetic modification of the association of paraquat and Parkinson's disease. Mov Disord. (13):1652-8.]
-
Paul KC, Sinsheimer JS, Cockburn M, Bronstein JM, Bordelon Y, Ritz B. 2017. Organophosphate pesticides and PON1 L55M in Parkinson's disease progression. Environ Int. 107:75-81. [Abstract Paul KC, Sinsheimer JS, Cockburn M, Bronstein JM, Bordelon Y, Ritz B. 2017. Organophosphate pesticides and PON1 L55M in Parkinson's disease progression. Environ Int. 107:75-81.]
-
Lee PC, Bordelon Y, Bronstein J, Ritz B. 2012. Traumatic brain injury, paraquat exposure, and their relationship to Parkinsonâs disease. Neurology. 79(20):2061-6. [Abstract Lee PC, Bordelon Y, Bronstein J, Ritz B. 2012. Traumatic brain injury, paraquat exposure, and their relationship to Parkinsonâs disease. Neurology. 79(20):2061-6.]
-
Lee PC, Bordelon Y, Bronstein J, Sinsheimer JS, Farrer M, Ritz B. 2015. Head injury, α-synuclein genetic variability and Parkinson's disease. Eur J Neurol. (5):874-8. [Abstract Lee PC, Bordelon Y, Bronstein J, Sinsheimer JS, Farrer M, Ritz B. 2015. Head injury, α-synuclein genetic variability and Parkinson's disease. Eur J Neurol. (5):874-8.]
-
Calderón-Garcidueñas L, Solt AC, HenrÃquez-Roldán C, Torres-Jardón R, Nuse B, Herritt L, Villarreal-Calderón R, Osnaya N, Stone I, GarcÃa R, Brooks DM, González-Maciel A, Reynoso-Robles R, Delgado-Chávez R, Reed W. 2008. Long-term air pollution exposure is associated with neuroinflammation, an altered innate immune response, disruption of the blood-brain barrier, ultrafine particulate deposition, and accumulation of amyloid beta-42 and alpha-synuclein in children and young adults. Toxicol Pathol. (2):289-310. [Abstract Calderón-Garcidueñas L, Solt AC, HenrÃquez-Roldán C, Torres-Jardón R, Nuse B, Herritt L, Villarreal-Calderón R, Osnaya N, Stone I, GarcÃa R, Brooks DM, González-Maciel A, Reynoso-Robles R, Delgado-Chávez R, Reed W. 2008. Long-term air pollution exposure is associated with neuroinflammation, an altered innate immune response, disruption of the blood-brain barrier, ultrafine particulate deposition, and accumulation of amyloid beta-42 and alpha-synuclein in children and young adults. Toxicol Pathol. (2):289-310.]
-
Liu R, Young MT, Chen JC, Kaufman JD, Chen H. 2016. Ambient air pollution exposures and risk of Parkinson disease. Environ Health Perspect; doi:10.1289/EHP135 [Abstract Liu R, Young MT, Chen JC, Kaufman JD, Chen H. 2016. Ambient air pollution exposures and risk of Parkinson disease. Environ Health Perspect; doi:10.1289/EHP135]
-
Liu R, Guo X, Park Y, Huang X, Sinha R, Freedman ND, Hollenbeck AR, Blair A, Chen H. 2012. Caffeine intake, smoking, and risk of Parkinson disease in men and women. Am J Epidemiol 175(11):1200-1207. [Abstract Liu R, Guo X, Park Y, Huang X, Sinha R, Freedman ND, Hollenbeck AR, Blair A, Chen H. 2012. Caffeine intake, smoking, and risk of Parkinson disease in men and women. Am J Epidemiol 175(11):1200-1207.]
-
Cruz-Hernandez A, Agim ZS, Montenegro PC, McCabe GP, Rochet JC, Cannon JR. 2018. Selective dopaminergic neurotoxicity of three heterocyclic amine subclasses in primary rat midbrain neurons. Neurotoxicology. 65:68-84 [Abstract Cruz-Hernandez A, Agim ZS, Montenegro PC, McCabe GP, Rochet JC, Cannon JR. 2018. Selective dopaminergic neurotoxicity of three heterocyclic amine subclasses in primary rat midbrain neurons. Neurotoxicology. 65:68-84]
-
Evatt ML, DeLong MR, Kumari M, Auinger P, McDermott MP, Tangpricha V. 2011. Parkinson study group DATATOP investigators: High prevalence of hypovitaminosis D status in patients with early Parkinsonâs disease. Arch Neurol 68(3):314-319. [Abstract Evatt ML, DeLong MR, Kumari M, Auinger P, McDermott MP, Tangpricha V. 2011. Parkinson study group DATATOP investigators: High prevalence of hypovitaminosis D status in patients with early Parkinsonâs disease. Arch Neurol 68(3):314-319.]
-
Gatto NM, Paul KC, Sinsheimer JS, Bronstein JM, Bordelon Y, Rausch R, Ritz B. 2016. Vitamin D receptor gene polymorphisms and cognitive decline in Parkinson's disease. J Neurol Sci. 370:100-106. [Abstract Gatto NM, Paul KC, Sinsheimer JS, Bronstein JM, Bordelon Y, Rausch R, Ritz B. 2016. Vitamin D receptor gene polymorphisms and cognitive decline in Parkinson's disease. J Neurol Sci. 370:100-106.]
-
Xu Q, Park Y, Huang X, Hollenbeck A, Blair A, Schatzkin A, Chen H. 2010. Physical activities and future risk of Parkinson disease. Neurology 75(4):341-348. [Abstract Xu Q, Park Y, Huang X, Hollenbeck A, Blair A, Schatzkin A, Chen H. 2010. Physical activities and future risk of Parkinson disease. Neurology 75(4):341-348.]
-
Paul KC, Chuang YH, Shih IF, Keener A, Bordelon Y, Bronstein JM, Ritz B. 2019. The association between lifestyle factors and Parkinson's disease progression and mortality. Mov Disord. 34(1):58-66. [Abstract Paul KC, Chuang YH, Shih IF, Keener A, Bordelon Y, Bronstein JM, Ritz B. 2019. The association between lifestyle factors and Parkinson's disease progression and mortality. Mov Disord. 34(1):58-66.]
-
Harischandra DS, Rokad D, Neal ML, Ghaisas S, Manne S, Sarkar S, Panicker N, Zenitsky G, Jin H, Lewis M, Huang X, Anantharam V, Kanthasamy A, Kanthasamy AG. 2019. Manganese promotes the aggregation and prion-like cell-to-cell exosomal transmission of alpha-synuclein. Sci Signal 12(572). [Abstract Harischandra DS, Rokad D, Neal ML, Ghaisas S, Manne S, Sarkar S, Panicker N, Zenitsky G, Jin H, Lewis M, Huang X, Anantharam V, Kanthasamy A, Kanthasamy AG. 2019. Manganese promotes the aggregation and prion-like cell-to-cell exosomal transmission of alpha-synuclein. Sci Signal 12(572).]
-
Searles Nielsen S, Warden MN, Camacho-Soto A, Willis AW, Wright BA, Racette BA. 2017. A predictive model to identify Parkinson disease from administrative claims data. Neurology 89(14):1448â1456. [Abstract Searles Nielsen S, Warden MN, Camacho-Soto A, Willis AW, Wright BA, Racette BA. 2017. A predictive model to identify Parkinson disease from administrative claims data. Neurology 89(14):1448â1456.]
-
Roede JR, Uppal K, Park Y, Lee K, Tran V, Walker D, Strobel FH, Rhodes SL, Ritz B, Jones DP. 2013. Serum metabolomics of slow vs. rapid motor progression Parkinsonâs disease: A pilot study. PLoS ONE 8(10): e77629. [Abstract Roede JR, Uppal K, Park Y, Lee K, Tran V, Walker D, Strobel FH, Rhodes SL, Ritz B, Jones DP. 2013. Serum metabolomics of slow vs. rapid motor progression Parkinsonâs disease: A pilot study. PLoS ONE 8(10): e77629.]
-
Meng C, Zhou J, Papaneri A, Peddada T, Xu K, Cui G. Spectrally Resolved Fiber Photometry for Multi-component Analysis of Brain Circuits. Neuron. 2018. Spectrally resolved fiber photometry for multi-component analysis of brain circuits. Neuron 98(4):707â717.e4. [Abstract Meng C, Zhou J, Papaneri A, Peddada T, Xu K, Cui G. Spectrally Resolved Fiber Photometry for Multi-component Analysis of Brain Circuits. Neuron. 2018. Spectrally resolved fiber photometry for multi-component analysis of brain circuits. Neuron 98(4):707â717.e4.]
Further Reading
Stories from the Environmental Factor (NIEHS Newsletter)
- Environmental exposures and Parkinson’s disease: connecting the dots (December 2022)
- Mitochondrial Damage Likely a Cause, Not a Consequence, of Parkinson’s (December 2021)
- Inhaled Paraquat Enters Brain, Impairs Sense of Smell in Male Mice (February 2021)
- Parkinson’s Driven by Inflammation, Genetics, and the Environment (February 2020)
Press Releases
- Medical History Can Point to Earlier Parkinson’s Disease Diagnosis (Sept. 15, 2017)
- NIH Scientists Find Six New Genetic Risk Factors for Parkinson's (July 27, 2014)
Additional Resources
- Environmental Neuroscience: Advancing the Understanding of How Chemical Exposures Impact Brain Health and Disease – Proceedings of a 2020 workshop published by the National Academies Press.
- MedlinePlus: Parkinson’s Disease – Information from the National Library of Medicine.
- Parkinson's Disease – Information from the National Institute on Aging of NIH.
- Parkinson’s Disease Research, Education and Clinical Centers – Information from the U.S. Department of Veterans Affairs.
- Talking to Your Doctor - Resources from NIH – Clear and honest communication between you and your physician can help you both make smart choices about your health. Ask questions to make sure you understand your diagnosis, treatment, and recovery.
Related Health Topics
This content is available to use on your website.
Please visit NIEHS Syndication to get started.

